Tungsten-Discovery, Properties, And Applications
Tungsten, also known as wolfram, is a chemical element with the symbol ‘W’ and atomic number 74. It is a rare metal that has a grayish-white color and is known for its high melting point, strength, and hardness. Due to these unique properties, tungsten has various industrial applications and is often used in high-stress environments.
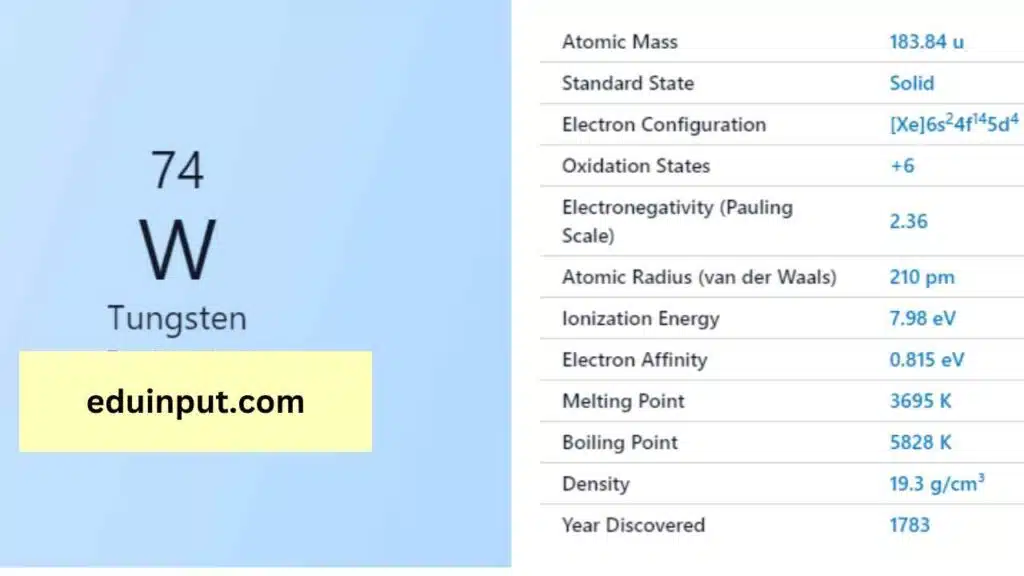
| Property | Value |
| Name | Tungsten |
| Symbol | W |
| Atomic number | 74 |
| Relative atomic mass (Ar) | Group in the periodic table |
| Standard state | Solid at 298 K |
| Appearance | Greyish white, lustrous |
| Classification | Metallic |
| Period in the periodic table | 6 |
| Group name | (none) |
| Block in the periodic table | 6 |
| Block in periodic table | d |
| Shell structure | 2.8.18.32.12.2 |
| CAS Registry | 7440-33-7 |
Discovery
Tungsten was discovered in 1781 by two Spanish chemists, Juan Jose and Fausto Elhuyar, who isolated the element from wolframite ore. However, it was not until 1847 that tungsten was first isolated in its pure form by a Swedish chemist, Carl Wilhelm Scheele.
Physical Properties
Tungsten is a dense metal with a high melting point of 3,422°C and a boiling point of 5,555°C. It has a density of 19.3 g/cm³, which makes it one of the heaviest metals. Tungsten also has the highest tensile strength of any metal and is extremely hard and durable.
Chemical Properties
Tungsten has an electron configuration of [Xe]4f^145d^46s^2 and is known to form compounds in a wide range of oxidation states, ranging from -2 to +6. Tungsten is often used in alloys, such as high-speed steel and tool steel, due to its high strength and hardness. Tungsten is also used in the production of filaments for incandescent light bulbs, electrical contacts, and X-ray tubes.
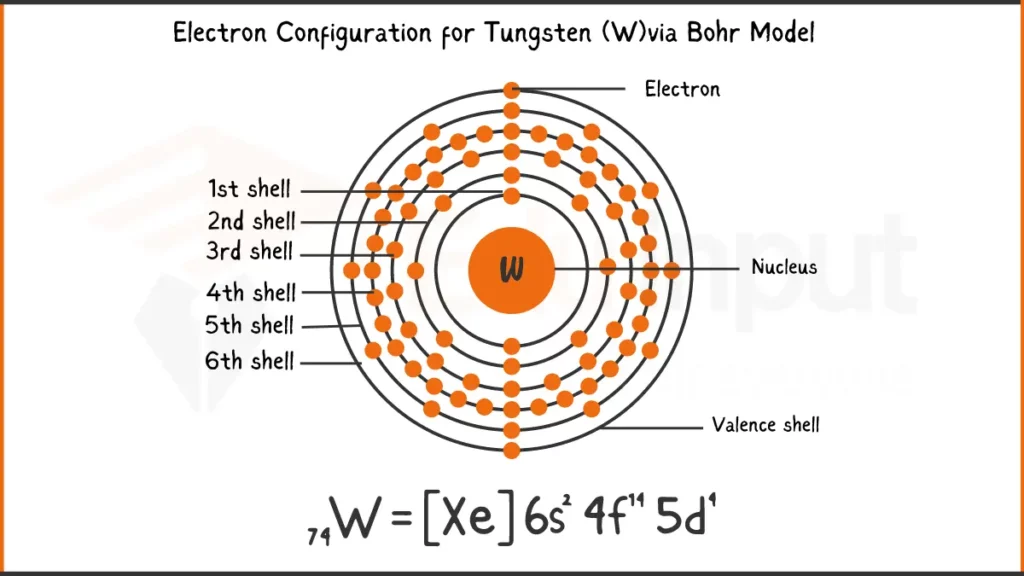
Electronic Configuration of Tungsten
Tungsten (W) possess 74 electrons. Its electronic configuration is [Xe]4f¹⁴5d⁴6s². This notation signifies that tungsten shares Xenon’s (Xe) inner electron structure, followed by 14 electrons in the 4f subshell, 4 in the 5d subshell, and 2 valence electrons in the 6s subshell.
Electronic Configuration of Tungsten via Bohr Model
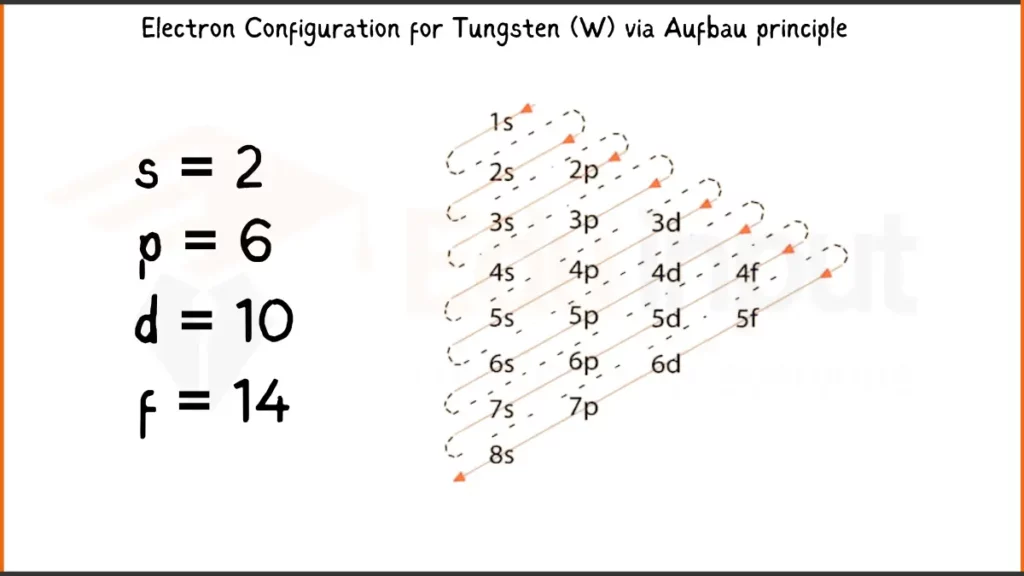
Electronic Configuration of Tungsten via Aufbau Principle
Facts
- Tungsten is the 74th element in the periodic table and is often referred to as ‘W’ due to its former name, wolfram.
- Tungsten is a very rare element, with a concentration of only 0.007% in the Earth’s crust.
- Tungsten is known for its high resistance to corrosion and is often used in chemical applications.
Applications
Tungsten has various industrial applications due to its unique properties. It is often used in alloys, such as high-speed steel and tool steel, which are used in cutting tools and machining applications. Tungsten is also used in the production of filaments for incandescent light bulbs, electrical contacts, and X-ray tubes due to its high melting point and strength. Tungsten is also used in the aerospace industry for its ability to withstand high temperatures and its resistance to corrosion.
Tungsten is a rare metal that is known for its high melting point, strength, and hardness. Due to its unique properties, tungsten has various industrial applications and is often used in high-stress environments. Tungsten is used in alloys, incandescent light bulbs, electrical contacts, and X-ray tubes, as well as the aerospace industry, highlighting its importance in modern technology.





Leave a Reply