Wave mechanical model of atom| Concept of atomic orbital
After the failure of Bohr’s atomic model, scientists were in search of a new model. This model was presented by Schrodinger. He has given an equation called the Schrodinger wave equation. This is called a quantum mechanical model. It indicates the
(i) Electrons have waves according to Broglie’s concept.
(ii) Heisenberg’s uncertainty principle is true.
(iii) The formation of the stationary energy levels for the electrons around the nucleus is also true.
Applications of wave mechanical model of atom
This wave mechanical model describes the electron as a three-dimensional wave in the electronic field of a positively charged nucleus. Schrodinger wave equation which is based on the concept of probability has the following applications:
(1) The equation is used to calculate the energy and wave function of particles moving in a single dimension.
(2) One can calculate the energy and wave function of a particle in a three-dimensional box.
(3) The equation is used to derive an expression for the energy of an electron in the hydrogen atom.
(4) The equation gave the concept of atomic orbital from the wave mechanical model.
CONCEPT OF ATOMIC ORBITAL
Definition
Three-dimensional regions of space around the nucleus of an atom, where there is a maximum probability of finding an electron with certain energy is called an atomic orbital.
Probability of finding the electron round the nucleus of 1s orbitals of H- atom and the radius of maximum probability (= 0.529Ao)
Actually, it is a space around the nucleus in an atom, where the electron spends most of its time while in motion.
| Orbit | Orbital |
| 1. It is a definite circular path at a definite distance from the nucleus in which the electrons move. 2. An orbit shows an exact position of an electron in an atom. 3. Orbit shows a certainty about the position of movement of an electron. 4. Orbital gives us the idea about the plane motion of the electron. 5. The maximum number of electrons in an orbit is given by 2n2. | 1. It is a space around the nucleus within which the probability of finding an electron with certain energy is maximum 2. Orbital does not specify the exact position of an electron in an atom. 3. According to the uncertainty principle, one is not sure about the movement of an electron in an orbit. 4. Orbital gives the three-dimensional motion of an electron. 5. An orbital cannot accommodate more than 2 electrons. |

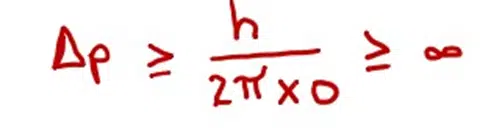


Leave a Reply