Why is Diffusion Faster in Air Than Water?
Diffusion is faster in air than in water because air has less density and viscosity than water. The lower density, viscosity, and molecular collisions in air allow for faster diffusion rates compared to liquids like water.

Also learn about: Why Is Diffusion Faster In Water Than in Agar?
Why is Diffusion Faster in Gases?
Reasons Why is Diffusion Faster in Air Than Water
Here are a few Why is Diffusion Faster in Air Than Water:
1. Lower Density
Air has lower density than water because air molecules are spaced further apart. This means that there are fewer air molecules in the way of diffusing particles. This reduces the number of collisions between diffusing particles and air molecules, which speeds up the diffusion process.
2. Lower Viscosity
Viscosity is a measure of how resistant a fluid is to flow. Air has lower viscosity than water, meaning that it flows more easily. This allows diffusing particles to move through air with less resistance, which speeds up the diffusion process.
3. Fewer Particle Collisions
The increased molecular spacing in air results in fewer collisions between diffusing particles and air molecules. Collisions slow down diffusion because they lose kinetic energy each time they collide. Fewer collisions mean that diffusing particles can retain more of their kinetic energy, which allows them to move faster and diffuse more quickly.
Example
Imagine that you are trying to diffuse a dye molecule through a cup of water. The dye molecule will diffuse slowly through the water because there are many water molecules in the way. The dye molecule will collide with water molecules frequently, which will slow down its movement.
Now imagine that you are trying to diffuse the same dye molecule through the air. The dye molecule will diffuse much faster through the air because there are fewer air molecules in the way. The dye molecule will collide with air molecules less frequently, which will allow it to move faster.
The same principle applies to all diffusing particles. The lower density of air allows diffusing particles to move more freely and collide with fewer other particles, which speeds up the diffusion process.

 written by
written by 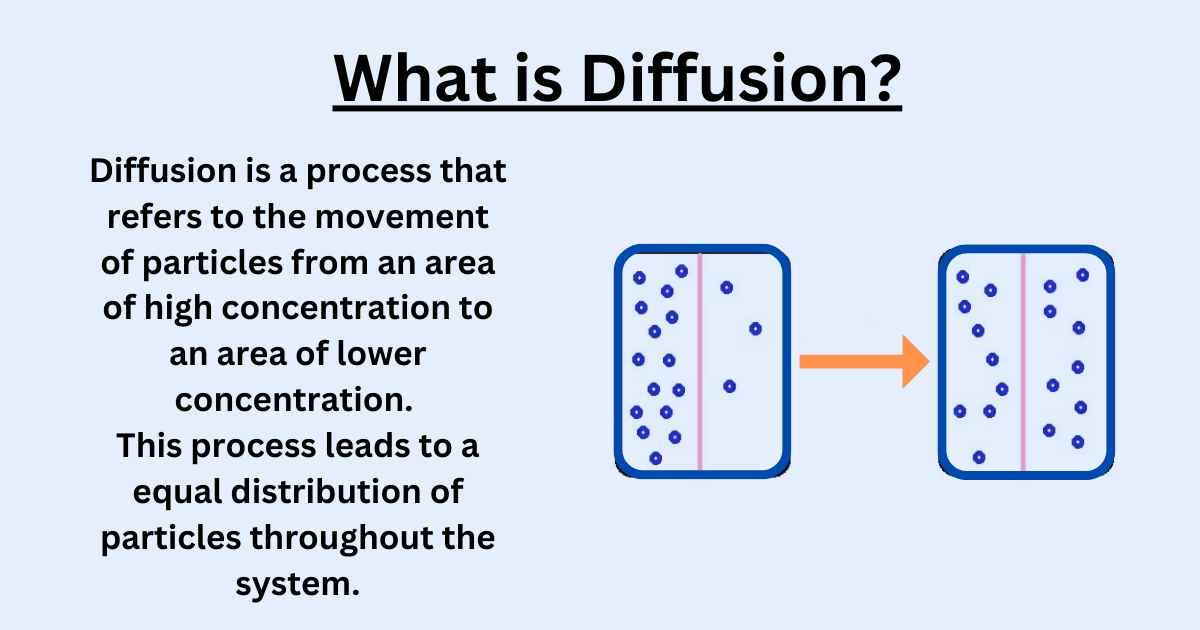





Leave a Reply