Thermochemistry-Reactions, Systems, Processes, and Aplications
Thermochemistry Definition
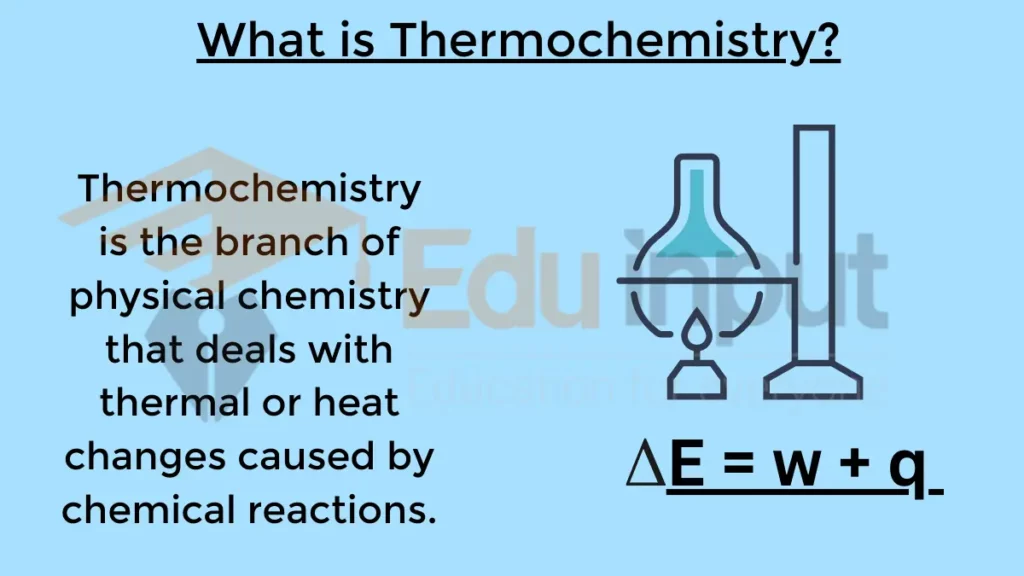
Thermochemistry is the branch of physical chemistry that deals with thermal or heat changes caused by chemical reactions. It is the science behind understanding how heat is either absorbed or released during a chemical transformation.
It studies the heat changes occurring in chemical reactions. It helps to understand the energetic aspects of chemistry.
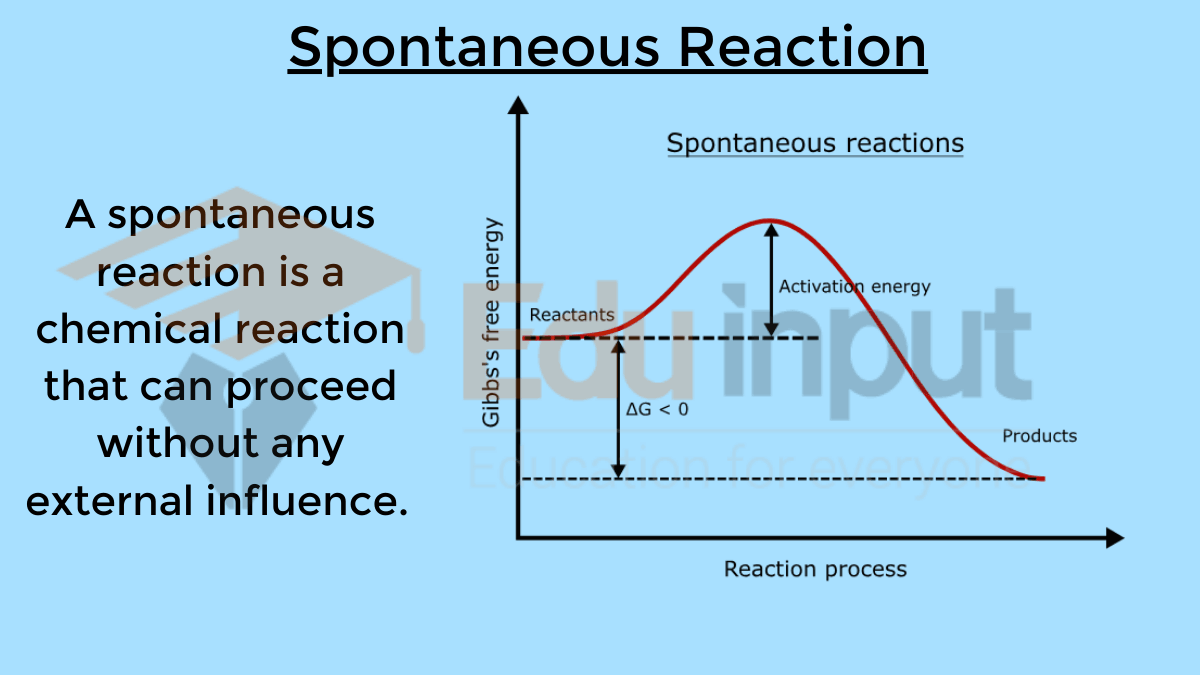
Also Read about Spontaneous Reactions
Read about Examples of Thermochemistry
Heat of Reaction
One of the fundamental concepts in thermochemistry is the heat of reaction. It is often symbolized as ΔH. This term refers to the amount of heat either evolved or absorbed in a chemical reaction. The sign of ΔH provides valuable information about the nature of the reaction:
1. When a reaction releases heat, ΔH is negative. This means that the reaction is exothermic, and it gives off energy in the form of heat. For instance:
Combustion of Wood
C6H10O5(s) + 6O2(g) → 6CO2(g) + 5H2O(g) + ΔH = -2808 kJ/mol
This equation illustrates that the combustion of cellulose in wood is an exothermic process, as indicated by the negative ΔH.
2. When a reaction absorbs heat, ΔH is positive. This indicates that the reaction is endothermic, and it requires an input of energy in the form of heat to proceed. For example:
Melting of Ice
H2O(s) → H2O(l) + ΔH = 6.01 kJ/mol
In this case, the melting of ice is an endothermic reaction, as denoted by the positive ΔH.
Energy Changes in Chemical Reactions
The energy changes in chemical reactions are attributed to the breaking and making of chemical bonds. When bonds are broken, energy is absorbed, and when new bonds are formed, energy is released.
In any chemical reaction, the energy required to break bonds is equivalent to the energy liberated during bond formation.
Consequently, the total energy of the reactants differs from that of the products, resulting in either an energy surplus (exothermic) or an energy deficit (endothermic) during the reaction.
Notably, heat changes are typically measured in the International System of Units (SI) using the unit of Joule (J) or its commonly used multiple, the kilojoule (kJ).
Thermochemical Reactions
Chemical reactions that involve heat changes are called thermochemical reactions. They are categorized into two primary types:
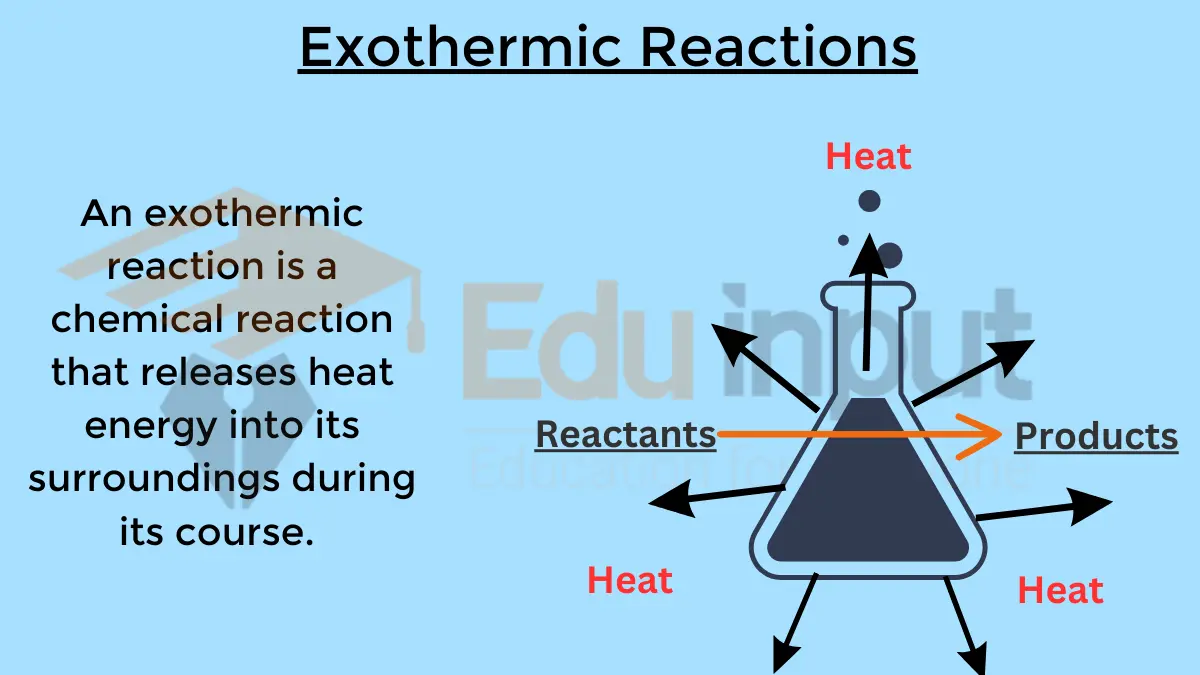
1. Exothermic Reactions
Exothermic reactions are characterized by the evolution of heat energy. These reactions release more energy than they absorb and have a negative ΔH. Exothermic reactions have a negative ΔH. Some examples include:
- C(s) + O2(g) → CO2(g) + ΔH = -393.7 kJ/mol
- 2H2(g) + O2(g) → 2H2O(g) + ΔH = -285 kJ/mol
- N2(g) + 3H2(g) → 2NH3(g) + ΔH = -41 kJ/mol
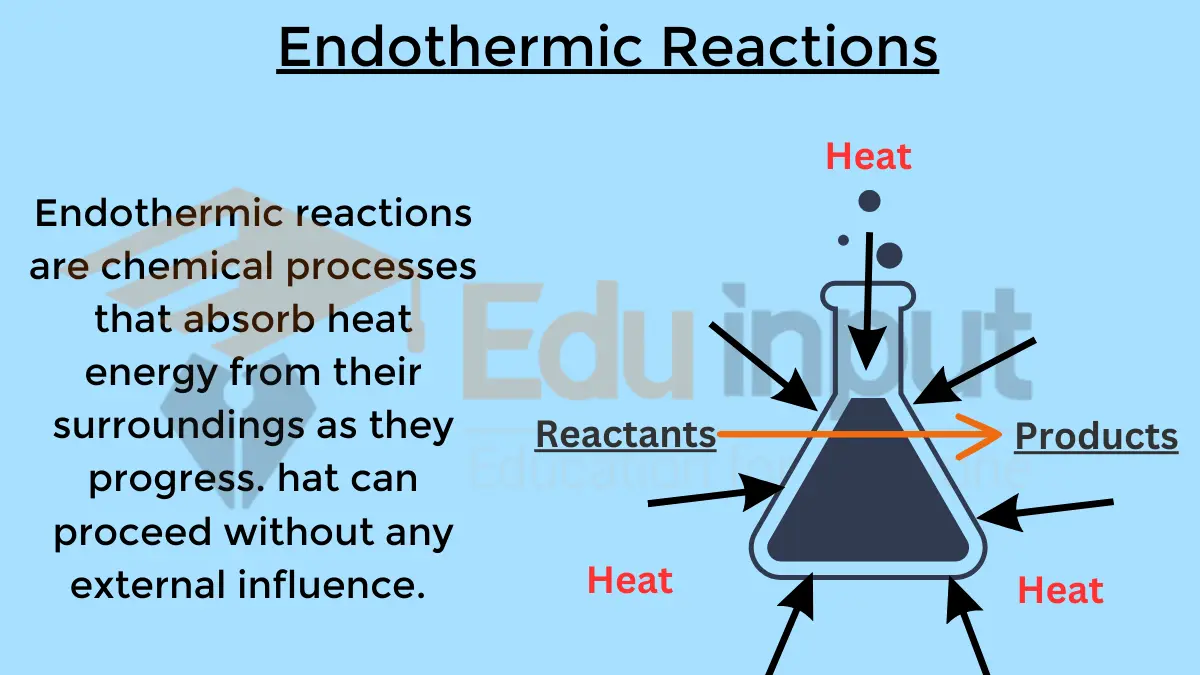
2. Endothermic Reactions
Endothermic reactions are characterized by the absorption of heat energy. These reactions require more energy input than they release and have a positive ΔH. Endothermic reactions have a positive ΔH. Some examples include:
- H2O(s) → H2O(l) + ΔH = 6.01 kJ/mol
- H2O(l) → H2O(g) + ΔH = 40.67 kJ/mol
- 6CO2(g) + 6H2O(l) → C6H12O6(aq) + 6O2(g) + ΔH = 2805 kJ/mol
- N2(g) + 3H2(g) → 2NH3(g) + ΔH = -92 kJ/mol
Systems in Thermochemistry
In thermochemistry, the term “system” refers to a specific portion of the universe under study or observation. It is used to define the boundaries of what is being analyzed or investigated in a thermodynamic process or chemical reaction.
There are three main types of systems in thermochemistry:
1. Closed System
A closed system is one that can exchange energy (typically in the form of heat) with its surroundings, but it does not exchange matter with the surroundings.
In other words, while energy can flow into or out of the system, the total mass of the system remains constant. A closed system is often represented as a container with impermeable walls that allow heat transfer.
2. Open System
An open system is one that can exchange both energy (heat) and matter with its surroundings. In this type of system, there can be a flow of substances into or out of the system in addition to heat transfer.
An example of an open system in thermochemistry could be a reaction vessel with a porous membrane that allows gases or liquids to flow in or out.
3. Isolated System
An isolated system is a conceptually idealized system in which neither matter nor energy (heat) can be exchanged with the surroundings.
In reality, perfectly isolated systems are rare, but they are useful for theoretical discussions and calculations in thermodynamics. An isolated system is totally isolated from its surroundings.
Processes in Thermochemistry
Thermochemistry involves various processes and measurements to study heat changes in chemical reactions. Here are some key processes in thermochemistry:
1. Calorimetry
Calorimetry is a fundamental process in thermochemistry. It involves the measurement of heat changes in a system by monitoring temperature changes. Calorimeters are devices designed for this purpose.
2. Heat Transfer
Thermochemistry explores how heat is transferred during chemical reactions. Heat can be transferred through conduction, convection, or radiation, and understanding these processes is vital for accurate measurements.
3. Enthalpy Change
Enthalpy (H) is a thermodynamic property that represents the heat content of a system at constant pressure. Thermochemists often calculate and analyze enthalpy changes (ΔH) to understand the heat flow in reactions.
4. Specific Heat Capacity
The specific heat capacity of a substance is the amount of heat required to raise the temperature of a unit mass of that substance by a certain amount. Thermochemistry involves the determination of specific heat capacities to understand how substances store and release heat.
5. Standard Conditions
Thermochemistry often refers to reactions taking place under standard conditions, which include a defined temperature (usually 298 K or 25°C) and pressure (usually 1 atmosphere). These conditions allow for consistent comparisons of heat changes between different reactions.
Instruments Used in Thermochemistry
To conduct experiments and measurements in thermochemistry, various instruments and apparatus are employed. Here are some commonly used instruments and equipment:
1. Calorimeter
A calorimeter is the central instrument in thermochemistry. It is designed to measure heat changes in chemical reactions. Different types of calorimeters, including bomb calorimeters for combustion reactions and coffee cup calorimeters for solution reactions, are used.
2. Thermometer
Thermometers are essential for measuring temperature changes in a calorimeter or during reactions. Digital thermometers and thermocouples are often used for precise temperature measurements.
3. Pressure Sensors
In some cases, pressure changes accompany heat changes during chemical reactions. Pressure sensors or gauges are used to monitor these changes.
4. Stirring Devices
Proper mixing of reactants is crucial for accurate measurements. Magnetic stirrers or mechanical stirrers are used to ensure uniform mixing of substances in a calorimeter.
5. Data Acquisition Systems
Modern thermochemistry experiments often involve data acquisition systems to record and analyze temperature, pressure, and other relevant data. These systems help automate data collection for increased precision.
6. Bomb Calorimeter
A bomb calorimeter is specifically designed to measure the heat of combustion in a sealed container (the bomb). It involves igniting a sample and measuring the temperature change in a calorimeter chamber surrounding the bomb.
7. Differential Scanning Calorimeter (DSC)
DSC is used to analyze phase transitions, reactions, and thermal properties of materials. It measures the difference in heat flow between a sample and a reference substance as a function of temperature.
8. Spectrophotometer
Spectrophotometers are used to study the absorption or emission of electromagnetic radiation during chemical reactions. They provide valuable information about energy changes in reactions involving light.
9. Gas Chromatograph (GC)
GC is employed to separate and analyze the components of a gaseous mixture. Thermochemistry often uses GC to study gas-phase reactions and determine reaction kinetics.
10. IR (Infrared) Spectrometer
IR spectrometers are used to analyze the infrared radiation absorbed by molecules. They help identify functional groups in compounds and study molecular vibrations, which can be related to heat changes.
List of Practical Experiments in Thermochemistry
In thermochemistry, various practical experiments and techniques are employed to study heat changes and energy transformations in chemical reactions and materials. Here is a list of common practical activities and experiments in thermochemistry:
- Calorimetry
- Heat of Formation
- Hess’s Law
- Enthalpy Change of Phase Transition
- Heat of Reaction and Stoichiometry
- Bond Dissociation Enthalpy
- Enthalpy of Combustion
- Reaction Kinetics
- Spontaneity and Gibbs Free Energy
- Adiabatic Process
- Thermal Analysis
- Environmental Thermochemistry
- Bio thermochemistry
- High-Temperature Thermochemistry
- Computational Thermochemistry
Applications of Thermochemistry
Here are some key applications of thermochemistry:
1. Chemical Engineering
Thermochemistry is fundamental in chemical engineering processes. Engineers use thermodynamic data to design and optimize chemical reactors, ensuring efficient production of chemicals, fuels, and pharmaceuticals. Understanding heat changes is essential for maintaining the safety and efficiency of industrial processes.
2. Calorimetry
Calorimetry, a branch of thermochemistry, is crucial for measuring heat changes accurately. It has applications in calorimeters used to determine the calorific values of fuels, study metabolic reactions in biology, and investigate the thermal properties of materials in materials science.
3. Environmental Science
Thermochemistry plays a key role in environmental studies. It helps assess the energy released or consumed during combustion processes, contributing to our understanding of air pollution, climate change, and the impact of emissions from vehicles and industrial facilities.
4. Food Industry
Thermochemistry is applied in the food industry to evaluate the energy content of food products. Calorimetry is used to determine the calorie content of foods, helping consumers make informed dietary choices.
5. Pharmaceuticals
Pharmaceutical researchers use thermochemistry to study drug reactions and stability. It aids in identifying the optimal conditions for drug synthesis and storage to maintain their effectiveness.
6. Energy Production
Thermochemistry is central to energy production, including the combustion of fossil fuels in power plants and engines. Understanding the heat of combustion is crucial for designing efficient energy conversion systems and addressing environmental concerns.
7. Materials Science
Thermochemistry helps characterize materials by studying their thermal behavior. This is vital in developing new materials for various applications, such as ceramics, polymers, and alloys.
8. Environmental Remediation
Thermochemical data assist in designing remediation processes for cleaning up contaminated sites. Techniques like thermal desorption and incineration rely on an understanding of heat changes to treat hazardous waste.
9. Metallurgy
In metallurgical processes, thermochemistry guides the extraction of metals from ores and their purification. It ensures that the desired chemical reactions occur at the right temperatures, leading to the production of high-quality metals.
10. Biology and Biochemistry
Thermochemistry is applied in studying biological reactions, including enzyme-catalyzed reactions and metabolic pathways. It helps elucidate how living organisms utilize and transform energy.
11. Nuclear Chemistry
In nuclear reactions, such as fission and fusion, thermochemistry helps determine the energy released or absorbed. This knowledge is essential for nuclear power generation and understanding nuclear reactions in astrophysics.
12. Chemical Analysis
Thermochemical techniques, like differential scanning calorimetry (DSC), are employed in chemical analysis to identify and quantify substances by measuring their heat-induced changes in physical properties.
13. Combustion Analysis
Thermochemistry is essential for combustion analysis, a technique used to determine the elemental composition of organic compounds by measuring the heat generated during combustion.
14. Environmental Monitoring
Thermochemistry contributes to environmental monitoring by assessing the energy changes involved in pollutant degradation, such as the breakdown of organic contaminants in soil or water.
Also Read: Difference between Spontaneous and Non-Spontaneous Reactions
Difference Between Endothermic And Exothermic Reactions



Leave a Reply