Fluorine-Discovery, Properties, And Applications
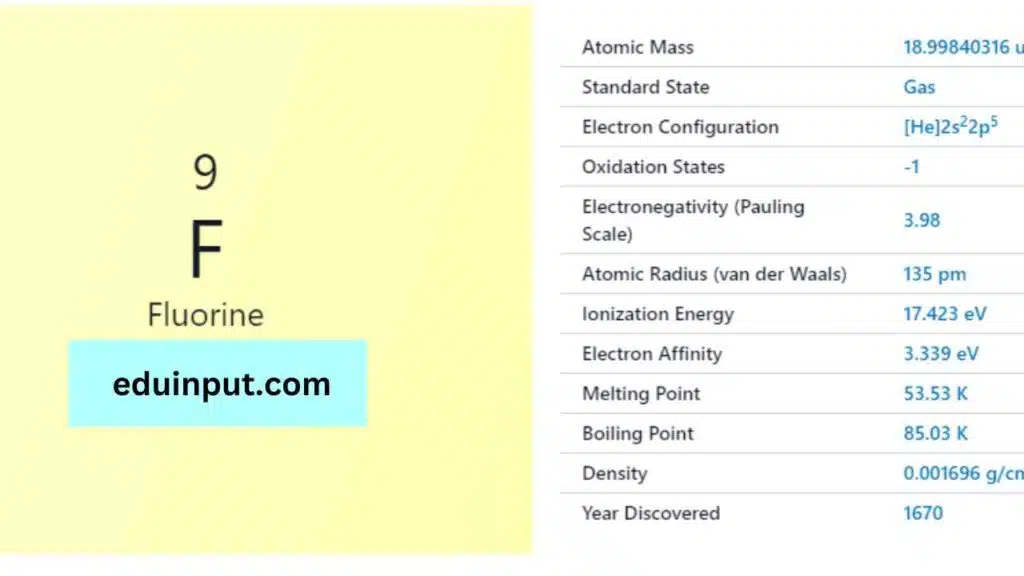
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists as a highly reactive pale yellow diatomic gas.
| Property | Value |
| Name | Fluorine |
| Symbol | F |
| Atomic number | 9 |
| Relative atomic mass (Ar) | 18.998403163 |
| Standard state | Gas at 298 K |
| Appearance | Pale yellow |
| Classification | Non-metallic |
| Period in the periodic table | 17 |
| Group name | Halogen |
| Block in the periodic table | 2 |
| Block in periodic table | p |
| Shell structure | 2.7 |
| CAS Registry | 7782-41-4 |
Discovery
Fluorine was discovered in 1886 by the French chemist Henri Moissan, who received the Nobel Prize in Chemistry in 1906 for his work on the isolation of fluorine.
Physical Properties
Fluorine is the lightest halogen and is a highly reactive gas. It is a pale yellow diatomic gas at standard conditions, and it readily forms compounds with most other elements.
Chemical Properties
Fluorine is the most reactive of all elements, and it is highly reactive with other elements, including noble gases. It readily forms compounds with most other elements, and it is used in the production of a wide range of chemicals.
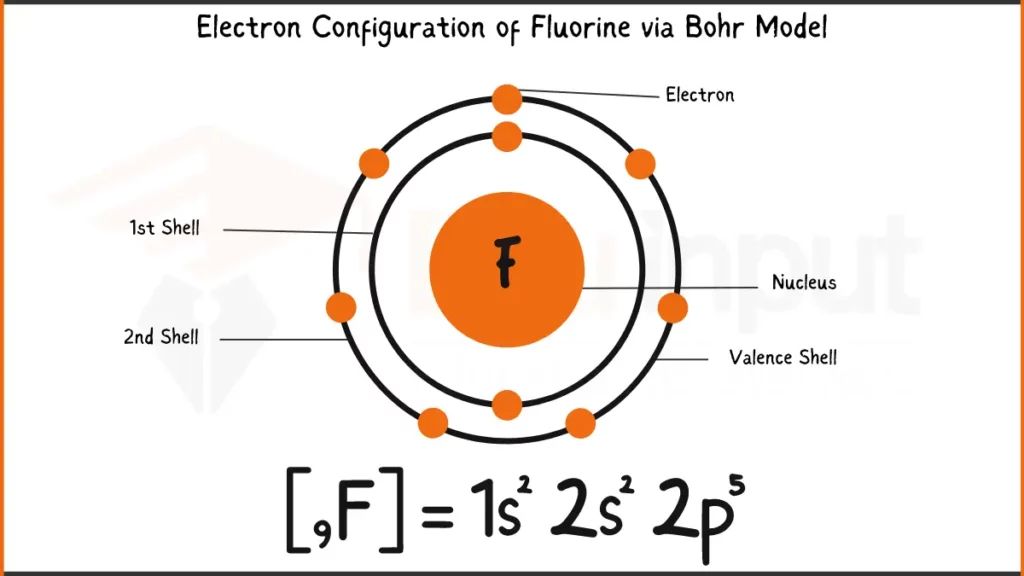
Electronic Configuration of Fluorine
Fluorine (F) has 9 electrons. Its electronic configuration is: 1s²2s²2p⁵. This signifies 2 electrons each in the 1s and 2s subshells, and 5 electrons in the 2p subshell.
Electronic Configuration of Fluorine via Bohr Model
Electronic Configuration of Fluorine via Aufbau Principle
Facts
- Fluorine is the most electronegative element and is highly reactive.
- Fluorine is used in the production of a wide range of chemicals, including refrigerants, plastics, and pharmaceuticals.
- Fluorine gas is extremely toxic and can cause severe burns and lung damage.
Applications
Fluorine is used in the production of a wide range of chemicals, including refrigerants, plastics, and pharmaceuticals. It is also used in the production of fluoride compounds for use in water treatment and toothpaste. In addition, fluorine is used in the nuclear industry to separate isotopes of uranium and to produce fluorine gas for use in plasma etching.






Leave a Reply