Molecular solids- formation, properties, crystal structure and uses
What are molecular solids?
Molecular solids are a type of crystalline solid in which the constituent particles are molecules held together by intermolecular forces.
Molecular solids definition
Unlike ionic or covalent network solids, where strong ionic bonds or covalent bonds bond the atoms throughout the entire structure, molecular solids consist of discrete molecules attracted to each other through weaker intermolecular forces.
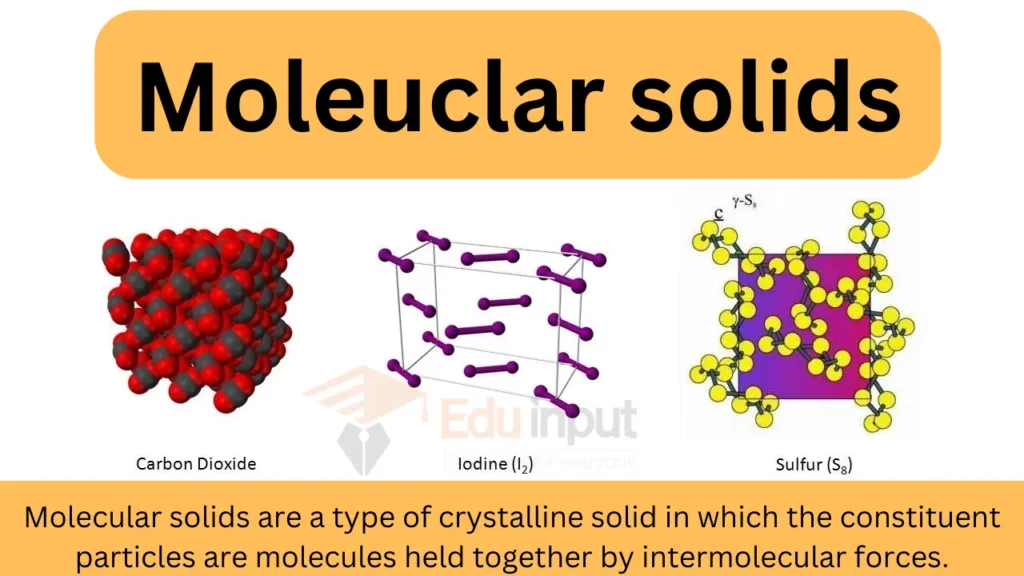
Building Blocks of Molecular Solids
Molecular solids are composed of individual units known as building blocks. These building blocks play a crucial role in determining the properties and functions of these solids. One common type of building block is the molecule, which is made up of two or more atoms chemically bonded together. These molecules can be simple, like water (H2O), or complex, like DNA.
The laws of chemistry govern the arrangement of atoms within a molecule and determine the molecule’s overall structure and properties. The atoms can be arranged in different configurations, giving rise to various types of molecules.
Some molecules have linear structures, while others have more complex geometries, such as cyclic or branched arrangements. These diverse arrangements of atoms enable the formation of a wide range of molecular solids with unique characteristics.
Arrangement of Molecules in molecular solids
Molecular solids, like ice and sugar, have patterns and structures formed by how their molecules arrange themselves. This arrangement is called a crystal lattice, and it’s crucial for determining the solid’s properties, like melting point and strength.
The molecules in a crystal lattice stick together due to intermolecular forces—these are attractive or repulsive forces between molecules. Forces like hydrogen bonding, dipole-dipole interactions, and van der Waals forces play a role.
For instance, hydrogen bonding happens when a hydrogen atom is attracted to a super-electronegative atom like oxygen or nitrogen. This strong interaction affects how the molecules arrange themselves in the crystal lattice. Understanding these forces helps us unravel molecular solids’ detailed patterns and structures.
Intermolecular Forces in molecular solids
Intermolecular forces play a vital role in determining the properties and behaviour of molecular solids. These forces arise due to the interactions between the positive and negative charges of different molecules.
One of the most common types of intermolecular forces is the dipole-dipole interaction. In molecules with polar covalent bonds, such as water (H2O) or ammonia (NH3), the electronegativity difference between the atoms leads to partial positive and negative charges. These partial charges attract the opposite charges on neighbouring molecules, creating a strong dipole-dipole interaction.
Another important intermolecular force is the London dispersion force, also known as van der Waals forces. These forces exist in all molecules, regardless of their polarity. In nonpolar molecules, there are temporary fluctuations in the electron distribution, resulting in the formation of instantaneous dipoles.
These temporary dipoles induce similar dipoles in neighbouring molecules, leading to a weak attraction between them. Although individually weak, the collective effect of London dispersion forces can significantly influence the physical properties of molecular solids, such as melting point and boiling point.
Crystal Lattices in Molecular Solids
Crystal lattices are like the blueprints of molecular solids, influencing how they behave. Picture them as organized 3D patterns made up of small identical building blocks called unit cells. These cells, marked by lattice points, act as guides for molecule arrangement.
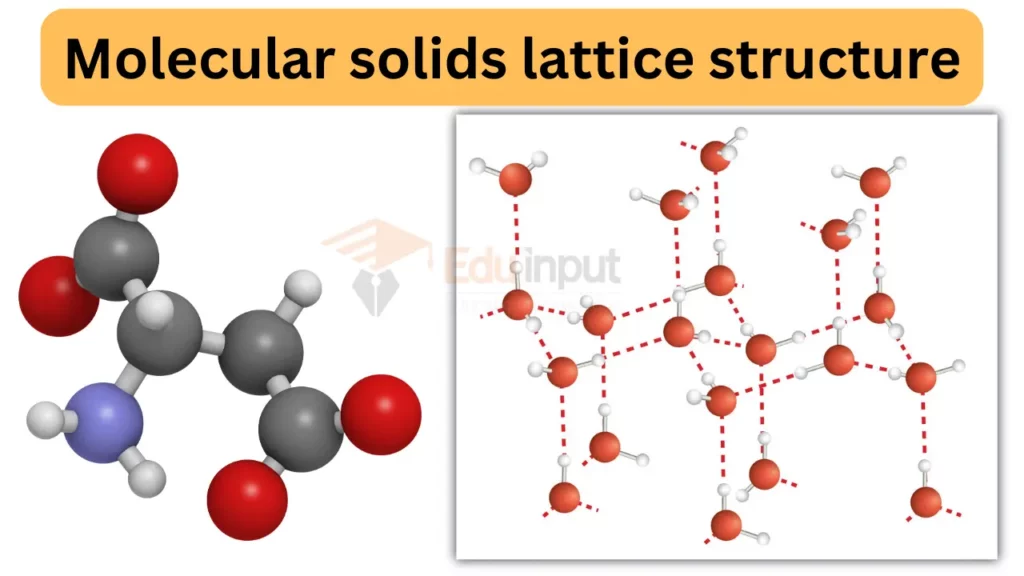
These lattice points are evenly spaced, keeping the crystal lattice stable. They decide the shape and size of the structure, giving it special properties like transparency or conductivity.
Understanding crystal lattices is key to studying molecular solids. Scientists use this knowledge to tap into the potential of these materials for various real-world uses, from electronics to medicine and energy. By figuring out how molecules are organized in these lattices, we unlock the secrets of these materials, paving the way for exciting applications.
Applications of molecular solids
Molecular solids have a variety of applications across different fields. Here are some notable applications, along with references to research papers for further reading:
- Pharmaceuticals: Molecular solids, especially in the form of polymorphs, solvates, and hydrates, are crucial in the pharmaceutical industry. They can significantly affect the solubility, stability, and bioavailability of drugs.
- Reference: Bernstein, J. (2002). Polymorphism in Molecular Crystals. Oxford University Press. This book provides a comprehensive overview of polymorphism in molecular crystals, which is essential for understanding the properties of pharmaceuticals.
- Organic Semiconductors: Organic semiconductors, often molecular solids, are used in electronic devices like organic light-emitting diodes (OLEDs), solar cells, and transistors. They offer advantages like flexibility and the potential for low-cost production.
- Reference: Anthony, J. E. (2008). Functionalized Acenes and Heteroacenes for Organic Electronics. Chemical Reviews, 108(1), 12–37. This paper discusses the development of organic semiconductors, focusing on acenes and heteroacenes.
- Optoelectronic Devices: Molecular solids are used in optoelectronic devices due to their ability to manipulate light. They are used in lasers, nonlinear optical devices, and photorefractive materials.
- Reference: Marder, S. R., Kippelen, B., Jen, A. K. Y., & Peyghambarian, N. (1997). Design and synthesis of chromophores and polymers for electro-optic and photorefractive applications. Nature, 388(6645), 845–851. This paper explores the design of chromophores and polymers for use in optoelectronic devices.
- Gas Storage and Separation: Porous molecular solids, like metal-organic frameworks (MOFs), are used for gas storage (e.g., hydrogen, methane) and separation due to their high surface area and tunable pore sizes.
- Reference: Li, H., Eddaoudi, M., O’Keeffe, M., & Yaghi, O. M. (1999). Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature, 402(6759), 276–279. This landmark paper discusses the design of MOFs for gas storage and separation.
- Thermal Insulators: Some molecular solids, due to their low thermal conductivity, are used as thermal insulators in various applications, ranging from electronics to building materials.
- Reference: Cui, Y., Xu, H., Yue, Y., Guo, K., Yu, J., & Chen, Z. (2015). A novel thermal insulating material based on aerogel: Preparation, characterization and application. Energy and Buildings, 87, 116–122. This paper discusses the development of a novel aerogel-based thermal insulating material.
- Catalysis: Certain molecular solids, particularly those with porous structures, serve as catalysts or catalyst supports in chemical reactions due to their high surface area and the ability to host active sites.
- Reference: Corma, A., García, H., & Llabrés i Xamena, F. X. (2010). Engineering metal organic frameworks for heterogeneous catalysis. Chemical Reviews, 110(8), 4606–4655. This review covers the use of MOFs in heterogeneous catalysis.





Leave a Reply