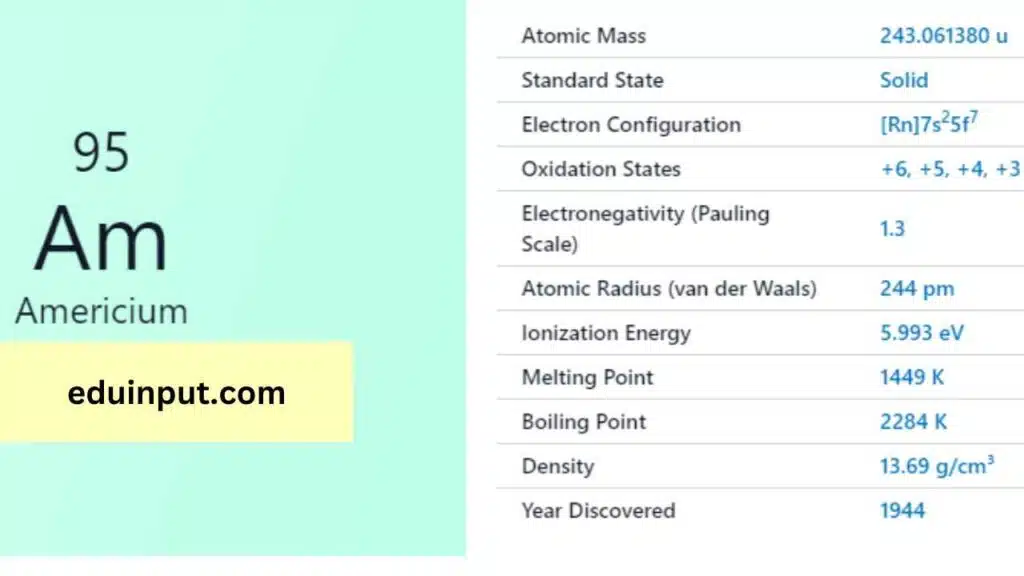
Americium-Discovery, Properties, And Applications
Americium is a radioactive chemical element with the symbol Am and atomic number 95. It is a synthetic element that was first produced in 1944 by Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso at the University of California, Berkeley.
| Property | Value |
| Name | Americium |
| Symbol | Am |
| Atomic number | 95 |
| Relative atomic mass (Ar) | Block in the periodic table |
| Standard state | Solid at 298 K |
| Appearance | Silvery white |
| Classification | Metallic |
| Group in periodic table | |
| Group name | Actinoid |
| Group in the periodic table | 7 (actinoid) |
| Period in the periodic table | f |
| Shell structure | 2.8.18.32.25.8.2 |
| CAS Registry | 7440-35-9 |
Physical Properties
- Americium is a silvery-white metal that slowly tarnishes in air.
- It is a dense metal with a melting point of 1173 K and a boiling point of 2607 K.
- Americium is paramagnetic, meaning that it is weakly attracted to a magnetic field.
Chemical Properties
- Americium is a highly reactive metal that reacts with oxygen, water vapor, and acids.
- It is a strong alpha emitter and has several isotopes, the most stable of which is Americium-243.
- Americium can be used as a fuel in some types of nuclear reactors.
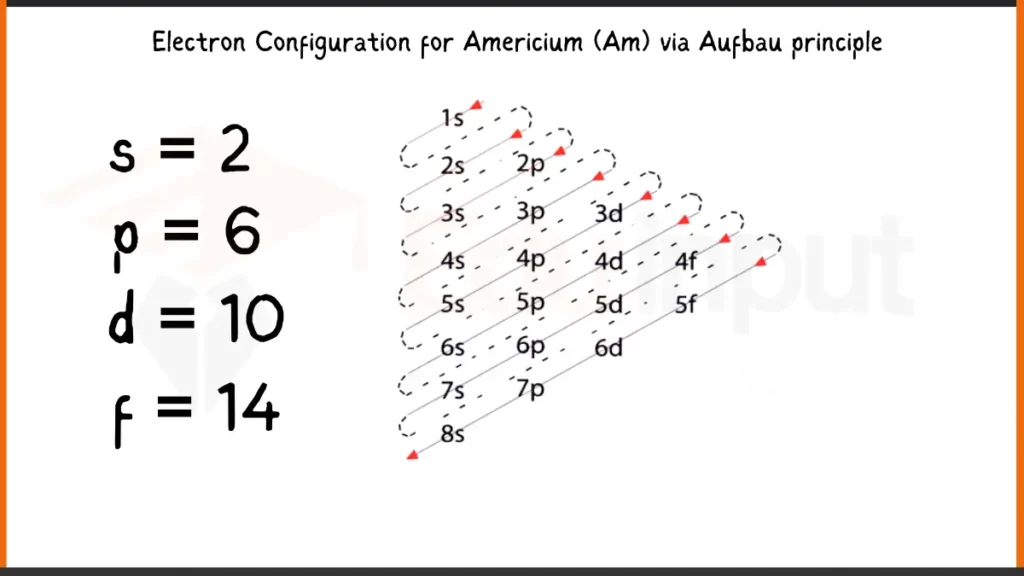
Electronic Configuration of Americium
Americium (Am), with 95 electrons, fills its orbitals based on the Aufbau principle. Its complete electron configuration is 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²4f⁷7s², showing the filling of the 5f subshell before 6d with seven electrons (5f⁷) due to specific energy level arrangements.
Electronic Configuration of Americium via Bohr Model
Electronic Configuration of Americium via Aufbau Principle
Facts
- Americium is one of the few elements that is named after a continent, in this case, the Americas.
- It has no known biological function and can be harmful to living organisms if ingested or inhaled.
- Americium was first used in commercial applications in the 1950s, primarily in smoke detectors.
Applications
- Americium is primarily used in smoke detectors, where it is used to ionize air particles and create a small electric current that is disrupted when smoke enters the detector.
- It is also used in some types of nuclear batteries, where the alpha particles emitted by Americium can be used to generate electricity.
- Americium has potential applications in nuclear weapons and nuclear reactors, but its use is heavily regulated due to its radioactivity.





Leave a Reply