Bromine-Discovery, Properties, And Applications
Bromine is a chemical element with the symbol Br and atomic number 35. It is in the halogen group and is the only non-metallic element that exists in liquid form at room temperature.
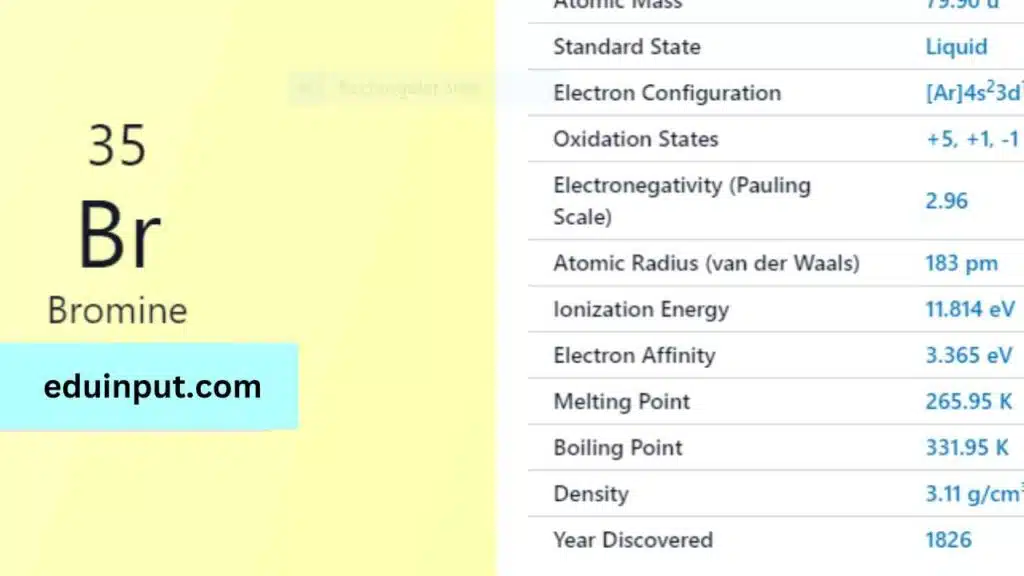
| Property | Value |
| Name | Bromine |
| Symbol | Br |
| Atomic number | 35 |
| Relative atomic mass (Ar) | 79.904 range: [79.901, 79.907] |
| Standard state | Liquid at 298 K |
| Appearance | Group in the periodic table |
| Classification | Non-metallic |
| Block in the periodic table | 17 |
| Group name | Halogen |
| Period in periodic table | 4 |
| Period in the periodic table | p |
| Shell structure | 2.8.18.7 |
| CAS Registry | 7726-95-6 |
Discovery
Bromine was discovered in 1826 by Antoine Balard, a French chemist. He discovered it while experimenting with the mineral salts found in the water of the Mediterranean Sea.
Physical Properties
Bromine is a reddish-brown liquid at room temperature with a pungent, irritating odor. It has a boiling point of 58.8°C and a melting point of -7.2°C. It is highly reactive and can form compounds with almost all other elements.
Chemical Properties
Bromine is a halogen and is highly reactive. It can react with almost all other elements, especially metals, to form compounds known as bromides. It can also react with organic compounds to form a wide range of products, including flame retardants, dyes, and pharmaceuticals.
Facts
- Bromine is the only non-metallic element that exists in liquid form at room temperature.
- Bromine is a powerful oxidizing agent and is often used in the production of flame retardants.
- Bromine is found in seawater and some mineral springs.
- Bromine is used in the production of pharmaceuticals, dyes, and photographic chemicals.
Applications
Bromine is widely used in the production of flame retardants, which are added to materials to make them less flammable. It is also used in the production of pharmaceuticals, dyes, and photographic chemicals.
Additionally, bromine compounds are used in water treatment to kill bacteria and other microorganisms. Bromine is also used in the petroleum industry to remove impurities from crude oil.






Leave a Reply