Covalent solids- types, formation, properties, crystal structure and uses
What are Covalent Solids?
Covalent solids are a type of solid material that is held together by covalent bonds.
Covalent solid definition
Covalent bonds form when atoms share electrons to achieve stability. In covalent solids, atoms are tightly connected, creating a strong and rigid structure. These bonds give covalent solids unique properties, such as high melting points and hardness.
Examples of covalent solids include diamond, graphite, and silicon carbide. These materials exhibit different atomic arrangements and bonding forces, resulting in variations in their physical and chemical properties.
The study of covalent solids provides valuable insights into material behavior. It has numerous applications in electronics, optoelectronics, and materials science.
Arrangement and bonding of atoms in covalent solids
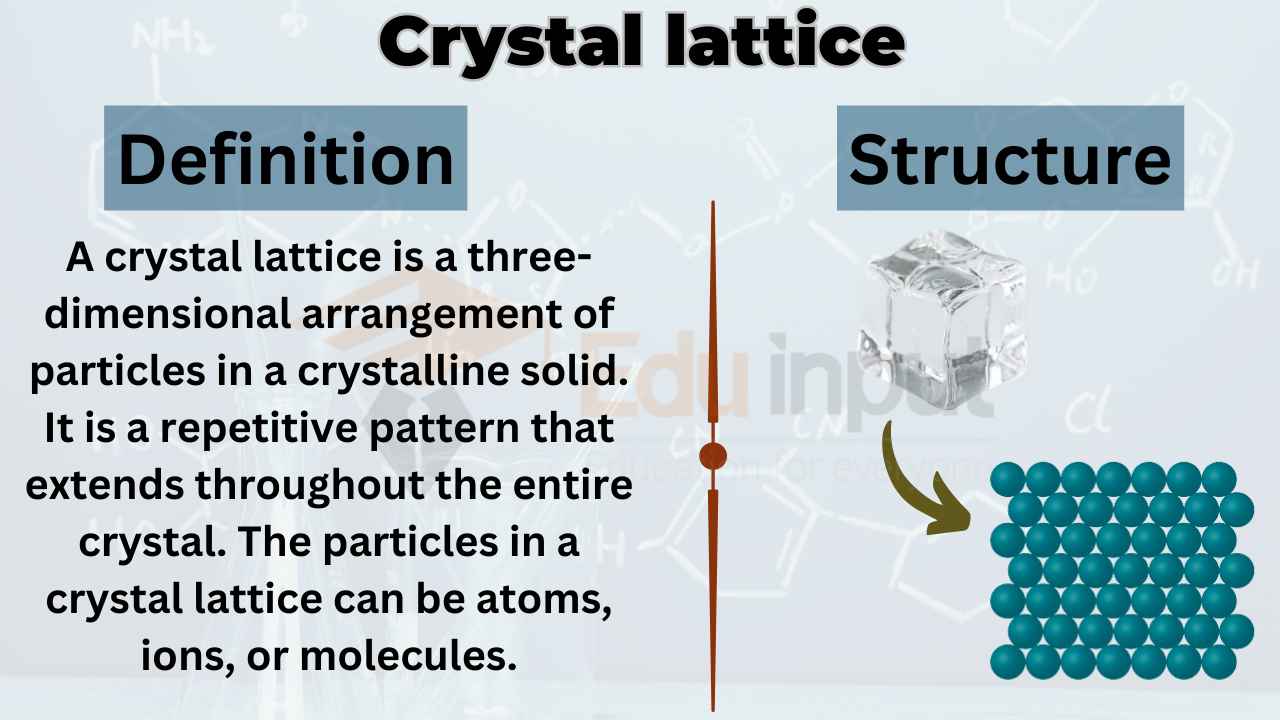
Covalent solids have a well-organized arrangement of atoms, which forms a structure known as a crystal lattice. This lattice gives solids stability and unique properties.
Atoms bond in these solids by overlapping their electron orbitals. This Property of solvents creates strong localized covalent bonds. As a result, the solid maintains its shape and resists deformation under pressure.
Covalent solids can be categorized into different types. These include molecular solids, network solids, and metallic solids. Each type has a distinct atomic arrangement that affects its properties.
Understanding the atomic structure and bonding in covalent solids is critical. It helps explain their characteristics and their use in industry and technology.
Types of Covalent Solids
There are two types of covalent solids: network covalent solids and molecular covalent solids.
Network Covalent Solids
Definition: Each atom is covalently bonded to neighboring atoms, forming a continuous three-dimensional network.
Characteristics:
- Solid and stable structures.
- High melting points.
- Generally, it is tough.
Example: Diamond
Each carbon atom is bonded to four other carbon atoms in a tetrahedral arrangement.
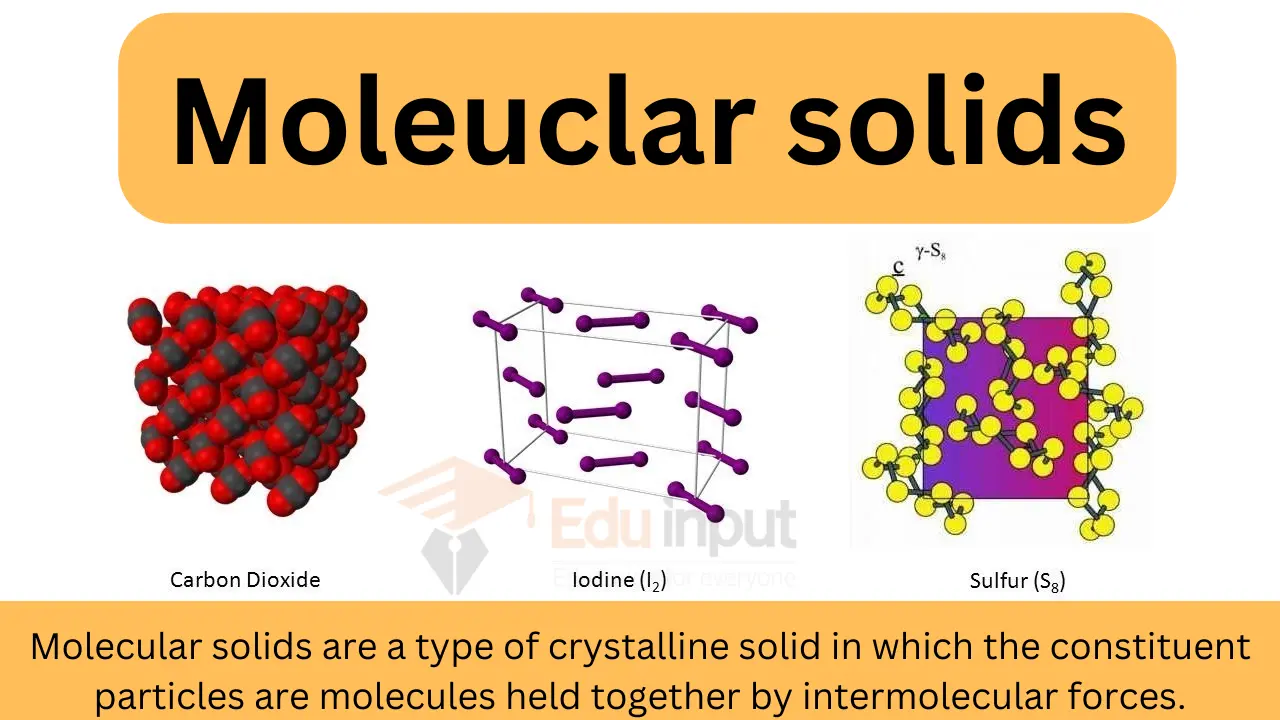
Molecular Covalent Solids
Definition: Discrete molecules are held together by intermolecular forces such as van der Waals or hydrogen bonding.
Characteristics:
- Individual molecules may be polar or nonpolar.
- Lower melting points compared to network covalent solids.
- Usually softer.
- Example: Solid Iodine
- Weak intermolecular forces hold individual iodine molecules together.
Properties of Covalent Solids
Covalent solids possess diverse physical and chemical properties.
High Melting and Boiling Points:
Strong covalent bonds within the solid require significant energy to break. This results in high stability, allowing covalent solids to remain solid at elevated temperatures.
Insolubility in Water and Polar Solvents:
- Covalent solids are generally insoluble in water and other polar solvents.
- Covalent bonds involve electron sharing, resulting in a balanced charge distribution.
- Less susceptible to interactions with polar molecules.
- Soluble in nonpolar solvents such as hexane or benzene.
Nature of Covalent Bonds
- Covalent bonds are formed by the sharing of electrons between atoms.
- This sharing leads to a balanced charge distribution in covalent solids.
- Contributes to their resistance to dissolution in polar solvents.
Versatile Solubility
- Covalent solids exhibit solubility in nonpolar solvents.
- This Property is utilized in various applications, from organic chemical synthesis to the production of advanced materials.
Stability at Elevated Temperatures
- The high melting and boiling points contribute to the stability of covalent solids.
- It allows them to maintain a solid state even when subjected to higher temperatures.
Applications in Advanced Materials
- Covalent solids find applications in the production of advanced materials.
- Their unique properties are valuable in organic chemistry and materials science industries.
Crystal Lattices in covalent solids
Covalent solids have different lattice structures based on their atoms and how they are arranged. One well-known type is the diamond structure. In diamonds, each carbon atom forms bonds with four other carbon atoms. This creates a tetrahedral shape. This arrangement makes diamonds very hard and transparent.
Another example is quartz. Quartz crystals are made of silicon and oxygen atoms arranged in a repeating pattern. These crystals have special properties called piezoelectricity, which means they can generate electricity when pressure is applied.
The diverse lattice structures in covalent solids give them various properties. This variety is important for many industries.
Bonding Forces in covalent solids
Covalent solids are held together by strong forces called intermolecular forces. These forces occur when the positively charged nuclei of nearby atoms interact with shared, negatively charged electrons. This creates a strong bond that forms a lattice structure in covalent solids, making them tough and resistant to heat.
A key intermolecular force in covalent solids is the dispersion force, or London dispersion force. This force arises from temporary changes in how electrons are arranged, which creates short-lived dipoles.
These temporary dipoles can influence nearby atoms or molecules, causing them to form their own temporary dipoles. This results in a weak attraction between them. The strength of the dispersion force depends on the size and shape of the atoms or molecules involved. Larger atoms tend to have stronger dispersion forces because they have more electrons and a bigger electron cloud.
The dispersion force is present in all covalent solids, regardless of their composition.
Thermal Conductivity
Covalent solids have distinct heat-related properties that set them apart from other solids. Their thermal conductivity—how well they conduct heat—varies based on structure.
The strength of covalent bonds, formed by electron sharing, significantly affects thermal conductivity. These strong bonds create a rigid structure, limiting atomic movement and heat transfer, which leads to low thermal conductivity in covalent solids.
In addition to low thermal conductivity, covalent solids often have high melting and boiling points. Breaking these strong bonds requires a lot of energy, providing stability at high temperatures.
Some covalent solids, like diamonds, exhibit unique thermal expansion properties. For example, diamonds expand very little when heated, making them highly resistant to thermal stress. This quality enhances their exceptional hardness and durability.ds very little when heated, making it highly resistant to thermal stress. This property contributes to the diamond’s exceptional hardness and durability.
Applications of covalent solids
| Application | Covalent Solid | Description |
| 1. Electronics | Silicon (Si) | Covalent solids like silicon are essential in the semiconductor industry for manufacturing electronic components such as transistors and integrated circuits. |
| 2. Cutting Tools | Diamond (C) | Diamond, a covalent solid, is the hardest known material and is widely used in cutting tools for machining, drilling, and grinding due to its exceptional hardness and durability. |
| 3. Insulating Materials | Silicon Dioxide (SiO2) | Covalent solids like SiO2 (quartz) are used as insulating materials in electronics and construction due to their stable structure and resistance to electrical conduction. |
| 4. High-Temperature Ceramics | Silicon Carbide (SiC) | Silicon carbide, a covalent solid, is used in the production of high-temperature ceramics and refractory materials due to its excellent thermal and mechanical properties. |
| 5. Structural Materials | Graphite (C) | Covalent solids like graphite are used as structural materials, lubricants, and in the production of composites due to their unique layered structure and good electrical conductivity. |
| 6. Dental Materials | Hydroxyapatite (Ca5(PO4)3OH) | Covalent solids like hydroxyapatite are used in dental applications, such as in the production of dental implants and bone grafts, due to their biocompatibility and strength. |
| 7. Optoelectronics | Gallium Nitride (GaN) | Covalent solids like GaN are crucial in optoelectronics for making LEDs (light-emitting diodes) and laser diodes, benefiting from their wide bandgap and high electron mobility. |
| 8. Adhesives | Polyethylene (C2H4)n | Covalent solids like polyethylene are used in the production of adhesives and various types of plastic due to their flexible and durable nature, making them suitable for bonding applications. |
| 9. Lubricants | Teflon (Polytetrafluoroethylene, PTFE) | Covalent solids like Teflon are used as lubricants and non-stick coatings due to their low friction properties and chemical inertness. |
| 10. Biomedical Applications | Polymethyl Methacrylate (PMMA) | Covalent solids like PMMA are used in biomedical applications, such as in the production of acrylic bone cement and contact lenses, due to their biocompatibility and transparency. |





Leave a Reply