Diffusion vs. Osmosis: Understanding the Differences
May 30, 2023
< />
< />
< />
< />
< />
< />
The main difference between diffusion and osmosis is that diffusion is the movement of particles from high to low concentration, while osmosis is the movement of solvent molecules to equalize concentration through a semipermeable membrane.
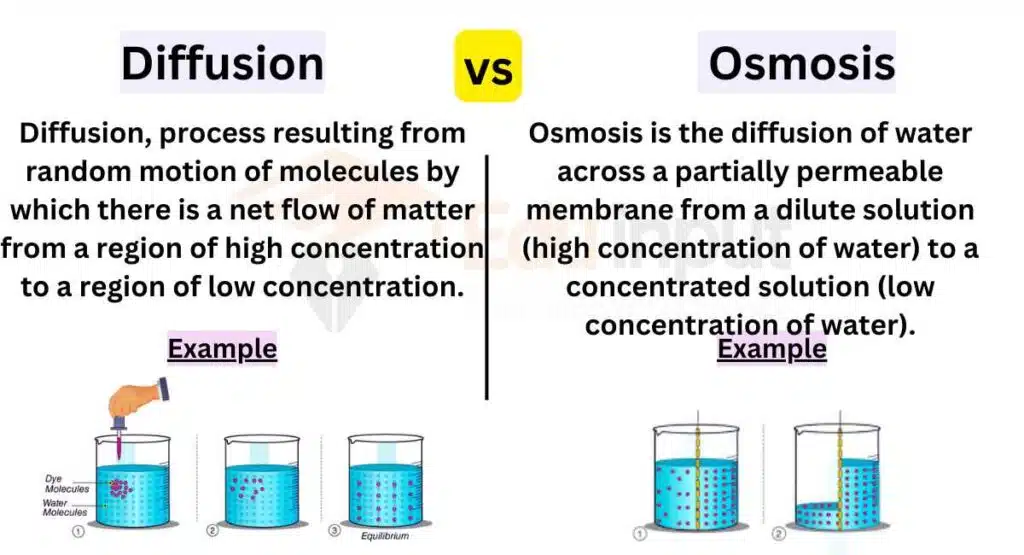
Diffusion vs. Osmosis
Here are the main Differences Between Diffusion and Osmosis:
| Factors | Diffusion | Osmosis |
|---|---|---|
| Definition | The movement of particles (solutes) from an area of high concentration to an area of low concentration until equilibrium is reached | The movement of water molecules across a selectively permeable membrane from an area of low solute concentration to an area of high solute concentration |
| Nature | Can involve the movement of any type of particles (solutes), including gases, liquids, and solids | Specifically refers to the movement of water molecules |
| Driving Force | Concentration gradient (difference in solute concentration) | Difference in solute concentration (osmotic gradient) |
| Membrane | Can occur through any type of membrane, including cell membranes and artificial barriers | Occurs specifically through a selectively permeable membrane |
| Direction | Occurs in all directions, from areas of high concentration to areas of low concentration until equilibrium is reached | Specifically occurs in the direction of higher solute concentration |
| Role in Living Systems | Essential for various biological processes, including nutrient absorption, gas exchange, and waste removal | Vital for maintaining water balance in cells and tissues, osmoregulation, and maintaining cell shape |
| Examples | Passive diffusion of oxygen and carbon dioxide across lung membranes | Movement of water into plant roots from the soil |
| Dependency | Not dependent on the presence of a solvent | Requires the presence of a solvent, typically water |
File Under:





Leave a Reply