Diffusion Rates-How Temperature, Medium, and Size Impact Movement
Diffusion rates (the speed at which molecules move) are influenced by key factors like temperature, medium, and molecule size. Higher temperatures increase particle movement, which leads to faster diffusion rates. Denser mediums like water offer more resistance, slowing diffusion compared to Diffusion in air.
Finally, the size of the molecule itself matters. Smaller molecules move more easily and have faster diffusion rates compared to larger, bulkier ones.
These factors do not act in isolation but rather interact with one another to influence the overall rate of diffusion. For example, an increase in temperature can enhance the rate of diffusion, but if the concentration gradient is shallow, the overall effect may be negligible. Understanding the interplay between these factors is crucial for comprehending and optimizing diffusion-dependent processes in various fields, including biology, chemistry, and materials science.
Factors Affecting Diffusion Rate
Here are the factors that impact the diffusion rate:
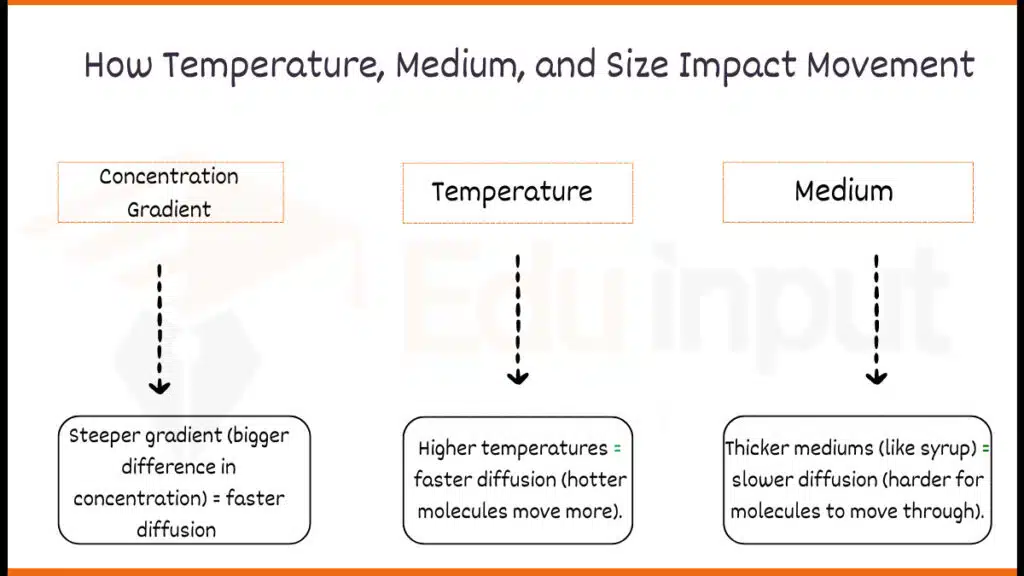
1. Concentration Gradient
The concentration gradient is the core driving force behind diffusion processes. It refers to the difference in the concentration of a particular substance between two regions. Molecules naturally tend to move from an area of higher concentration to an area of lower concentration, seeking to establish an equilibrium state.
The steeper the concentration gradient, the faster the rate of diffusion, as the molecules have a stronger driving force to move down the gradient.
2. Temperature
Temperature plays a significant role in influencing the rate of diffusion. According to the Arrhenius equation, an increase in temperature leads to an increase in the kinetic energy of molecules, which results in more frequent collisions and faster movement. Consequently, at higher temperatures, molecules diffuse more rapidly across a medium.
This principle is particularly relevant in biological systems, where temperature fluctuations can impact various diffusion-dependent processes, such as nutrient uptake, gas exchange, and cellular signaling.
3. Medium
The physical state and composition of the medium through which diffusion occurs can significantly affect the rate of this process. In gases, diffusion occurs more rapidly than in liquids or solids due to the greater average distance between molecules and their higher kinetic energy.
In liquids, the rate of diffusion is slower compared to gases but faster than in solids, as the molecules are more tightly packed but still have some mobility. In solids, diffusion is generally the slowest due to the rigid arrangement of atoms or molecules, which limits their movement.
The composition of the medium can influence diffusion rates. For example, in biological systems, the presence of membranes or barriers can restrict the movement of certain molecules, thereby slowing down the diffusion process.
4. Pressure
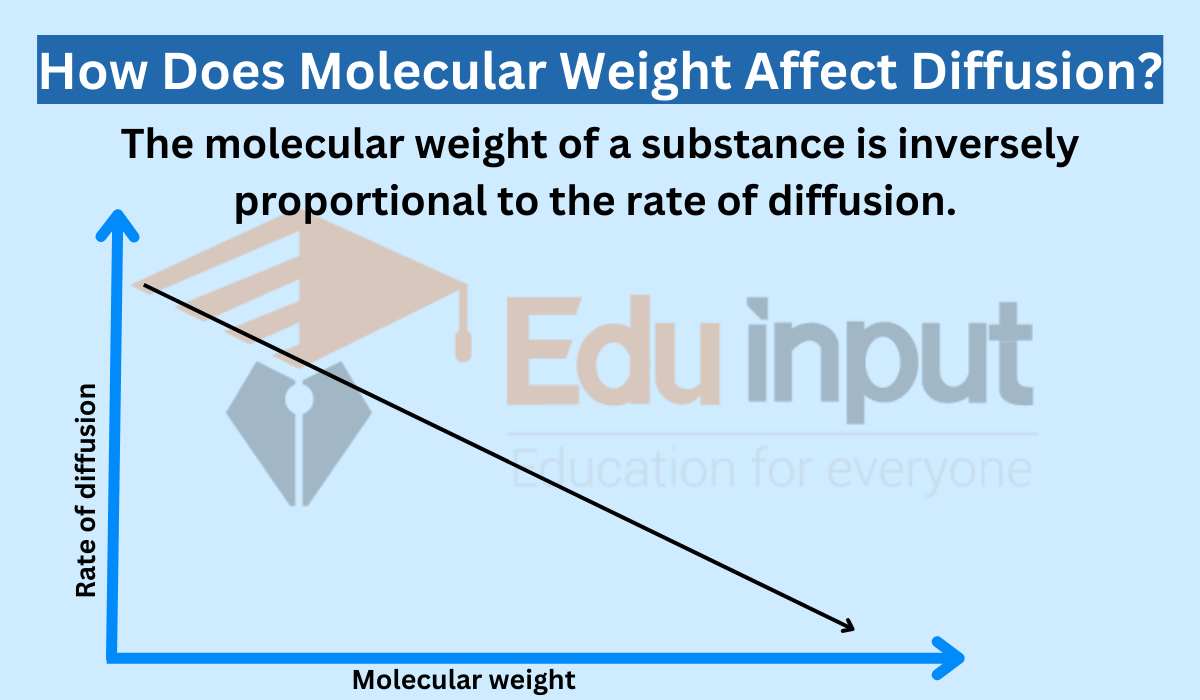
Pressure (particularly in gases) can impact the rate of diffusion. According to Graham’s law of diffusion, the rate of diffusion of a gas is inversely proportional to the square root of its molar mass. This means that lighter gases, such as hydrogen or helium, diffuse more rapidly than heavier gases, like carbon dioxide or oxygen, under the same conditions of temperature and pressure.
An increase in pressure can lead to an increase in the density of a gas, resulting in a higher concentration of molecules per unit volume. This higher concentration can drive the diffusion of molecules from regions of higher pressure to regions of lower pressure, a phenomenon known as effusion.
In biological systems, pressure differences can play a role in processes such as gas exchange in the lungs or the movement of gases across cell membranes.



Leave a Reply