HEISENBERG’S UNCERTAINTY PRINCIPLE-In depth explanation
Definition of HEISENBERG’S UNCERTAINTY PRINCIPLE
It is difficult to determine the positon as well as the momentum of the electron simultaneously.
According to Bohr’s theory, an electron is considered a material particle. The position and momentum of an electron cannot be determined with great accuracy. According to de Broglie, the electron has a wave associated with it. So the measurement of both parameters simultaneously is difficult.
In order to understand this principle, Let’s imagine that we throw some photons of X rays at the moving electron. These photons have a very short wavelength. The possibility for these photons to hit the electron is very bright.
So one can assess the position of the electron when the photons of X- rays reflects back. But this photon of very high energy of X- rays have disturbed the velocity of the electron and its momentum has changed. In other words, uncertainty in the momentum has been created.
In order to avoid the uncertainty in the momentum, we throw photons of visible light on the electron. These photons are weak as compared to the photon of X- rays. There are fewer chances for uncertainty in the momentum.
But due to the greater wavelength of this photon, the chance of hitting on the electron becomes very small, so the position becomes uncertain.
Mathematical expression for Heisenberg’s uncertainty principle
If the Δx represents the uncertainty of position and Δp represents the uncertainty of momentum for a subatomic particle like an electron, then
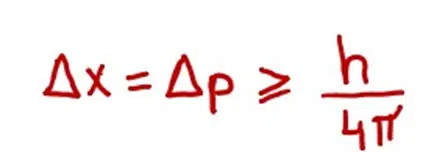
(> indicates equal to or greater than)
This relation is called uncertainty relation. According to this relation if Δx is small, Δp will be large and vice versa.
Hence if one quantity is measured accurately the other quantity is measured less accurately. This also means that the certainty of determination of one quantity introduces uncertainty for the determination of other quantity.
Above equation can be written as
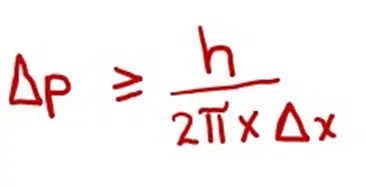
If Δx = 0 (we are sure about the position of the particle)
Then
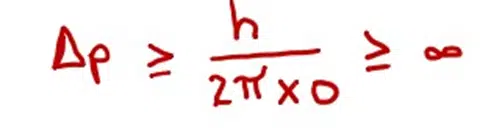
It means that the error in the determination of the momentum is infinite. In other words, the momentum of the particle cannot be measured, when we are sure about the position of the electron.



Leave a Reply