Ionization energy, definition, examples, significance, factors, periodic trend
Ionization Energy definition
The minimum amount of energy required to remove an electron from the valence shell of an isolated gaseous atom to form a positive ion is called ionization energy.
What is Ionization Energy?
This process results in the formation of a positively charged ion. The ionization energy is typically expressed in units such as kilojoules per mole (kJ/mol) or electron volts (eV). It is an important concept in the field of chemistry and plays a significant role in understanding the behavior of atoms.
How to calculate ionization energy
The general equation to calculate ionization energy is:
Ionization Energy (IE)
= ΔE = -E_final – (-E_initial)
Where:
- ΔE is the ionization energy (in energy units).
- E_final is the energy of the system after the electron has been removed.
- E_initial is the energy of the system before the electron was removed.
Here are three solved examples to illustrate how to calculate ionization energy:
Example 1: Ionization energy of hydrogen (H)
Let’s calculate the ionization energy of a hydrogen atom (H) in its ground state, where it has one electron.
- Determine the energy of the hydrogen atom in its initial state (E_initial):
The energy of a hydrogen atom can be calculated using the formula for the energy of an electron in a hydrogen atom: E_initial = -13.6 eV (since it’s the ground state energy of hydrogen) - Remove an electron to create a hydrogen cation (H+):
H → H+ + e- - Determine the energy of the hydrogen cation (H+) in its final state (E_final):
E_final = 0 eV (since it’s a singly ionized hydrogen cation with no electrons) - Calculate the ionization energy (ΔE):
ΔE = E_final – E_initial
ΔE = 0 eV – (-13.6 eV)
ΔE = 13.6 eV
So, the ionization energy of hydrogen is 13.6 electronvolts (eV).
Example 2: Ionization energy of helium (He)
Now, let’s calculate the ionization energy of helium, which has two electrons.
- Determine the energy of the helium atom in its initial state (E_initial):
E_initial = -54.4 eV (ground state energy of helium) - Remove one electron to create a helium cation (He+):
He → He+ + e- - Determine the energy of the helium cation (He+) in its final state (E_final):
E_final = -24.6 eV (energy of He+ with one electron removed) - Calculate the ionization energy (ΔE):
ΔE = E_final – E_initial
ΔE = (-24.6 eV) – (-54.4 eV)
ΔE = 29.8 eV
So, the ionization energy of helium is 29.8 eV.
Example 3: Ionization energy of lithium (Li)
Let’s calculate the ionization energy of lithium, which has three electrons.
- Determine the energy of the lithium atom in its initial state (E_initial):
E_initial = -198.0 kJ/mol (ground state energy of lithium) - Remove one electron to create a lithium cation (Li+):
Li → Li+ + e- - Determine the energy of the lithium cation (Li+) in its final state (E_final):
E_final = -520.2 kJ/mol (energy of Li+ with one electron removed) - Calculate the ionization energy (ΔE):
ΔE = E_final – E_initial
ΔE = (-520.2 kJ/mol) – (-198.0 kJ/mol)
ΔE = 322.2 kJ/mol
So, the ionization energy of lithium is 322.2 kilojoules per mole (kJ/mol).
Examples of ionization energy
H → H+ + e– ΔH= +1312 kJ/mol
Mg → Mg+ + e– ΔH= +738 kJ/mol
some other examples of ionization energies of elements
| Elements | Ionization energy (KJ/mol) |
| Helium (He) | 2372 kJ/mol |
| Sodium (Na) | 496 kJ/mol |
| Oxygen (O) | 1314 kJ/mol |
| Fluorine (F) | 1681 kJ/mol |
| Nitrogen (N) | 1402 kJ/mol |
Significance of Ionization Energy
Understanding ionization energy is crucial for several reasons:
Chemical Reactivity
The ease with which an atom loses its outer electrons influences its chemical reactivity. Elements with low ionization energy are more likely to form positive ions and engage in chemical reactions.
Periodic Trends
Ionization energy exhibits trends across the periodic table, providing insights into the atomic structure and behavior of elements.
Identification of Elements
Ionization energy patterns help identify elements and predict their chemical behavior.
Factors affecting ionization energy
The ionization energy of an atom depends upon the following factors:
The atomic radius of an atom
As the atomic radius increases, the nuclear pull for valence electrons decreases. As a result, less amount of energy is required to remove an electron from the valence shell. Thus with an increase in atomic size ionization energy decreases.
Nuclear charge or proton number of the atom
The greater the effective nuclear charge, the greater the pull for valence electrons. So, a greater amount of energy is required to remove tightly bound electrons.
Shielding effect of inner electrons
An increase in the shielding effect results in a decrease of nuclear attraction for valence electrons, so ionization energy decreases.
Nature of orbital
The arrangement of orbitals according to increasing ionization energy values is as under
s>p>d>f
Higher ionization energies
Second ionization energy
The amount of energy required to remove the second electron from a uni-positive ion to form a di-positive ion is called second ionization energy.
Mg+ → Mg+2 + e- ΔH= + 1450 kJ/mol
ionization energy values undergo an increase with the increase in the number of electrons to be removed. This is because the second electron is removed from a positively charged ion rather than a neutral atom. The dominant positive charge holds the electrons more tightly and thus further removal of electrons becomes more difficult. This is the reason the second ionization energy is greater than the first ionization energy.
Third ionization energy
The amount of energy required to remove the third electron from a di-positive ion to form a tri-positive ion is called the third ionization energy.
Mg+2 → Mg+3 + e- ΔH= + 7730 kJ/mol
Periodic trend
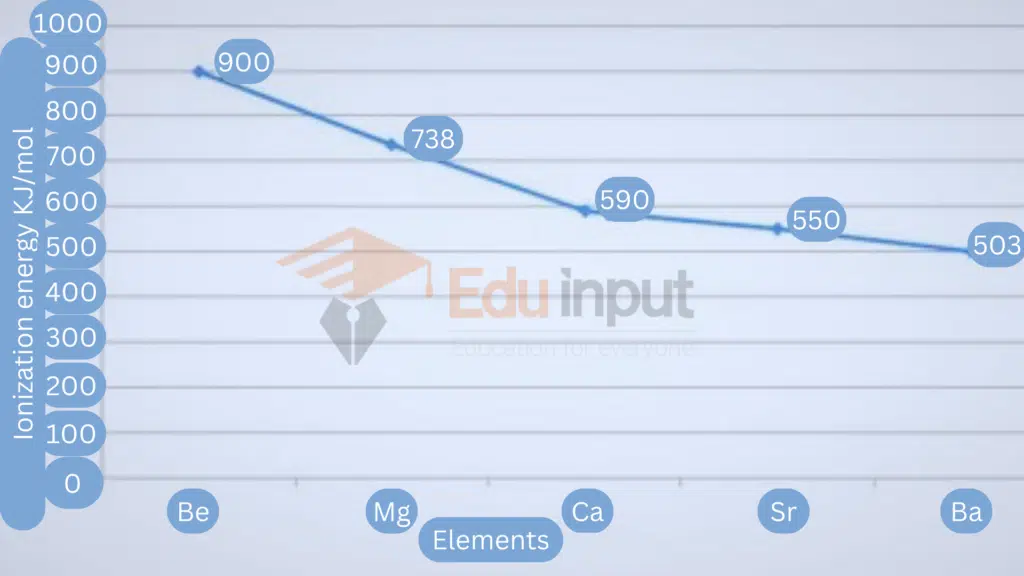
Variation in groups
Ionization energy values decrease from top to bottom in a group. This is because:
Atomic size increases due to the addition of new shells.
The shielding effect of inner shells increases.
As a result, effective nuclear attraction for valence shell electrons decreases and less amount of energy is required to remove the electron.
Variation in periods
Ionization energy values increase from left to right in a period. This is because of the following reasons:
There is no addition of new shells along the period, so electrons are added in the same shell.
Effective nuclear charge increases and the attractive forces between the nucleus and electrons increase.
The shielding effect almost remains constant.
Electrons are more tightly bound and difficult to remove.
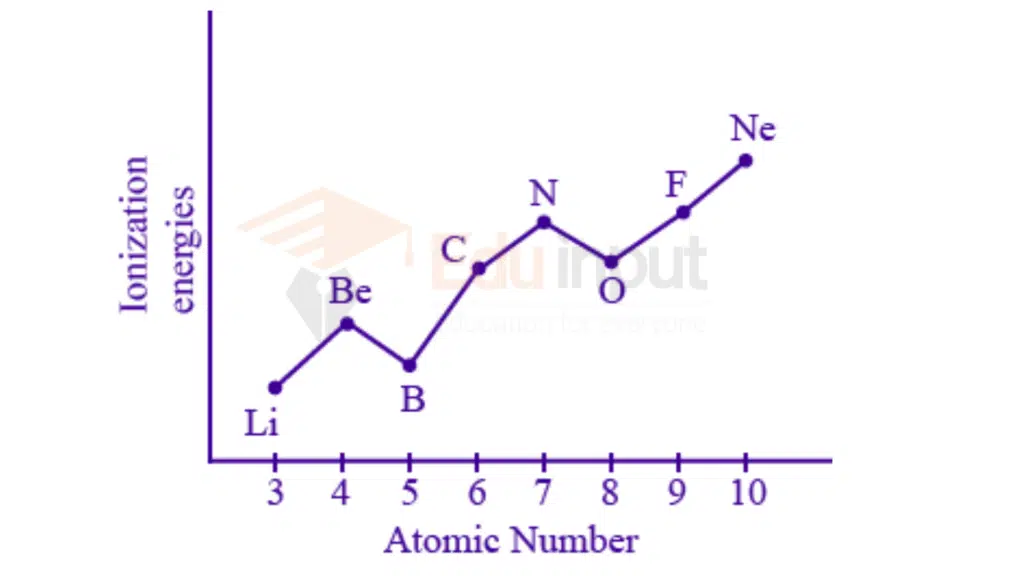
Abnormalities of ionization energies in periods
Following abnormalities in ionization energies have been noted.
IIIA group elements have less ionization energy values than IIA group elements.
Reasons: Completely filled orbitals are always more stable than partially filled orbitals. A greater amount of energy is required to remove an electron from stable orbitals. Since completely filled s-orbitals in the IIA group elements are more stable than partially filled p-orbitals of IIIA group elements. So IIIA group elements have less ionization energy values than IIA group elements
VIA group elements have less ionization energy values than VA group elements.
Reasons: Half-fully filled orbitals are always more stable. A greater amount of energy is required to remove an electron from stable orbitals. Since half-filled p-orbitals in the VA group elements are more stable than the p-orbitals of VIA group elements. So VIA group elements have less ionization energy values than VA group elements.





Leave a Reply