Molecular Orbitals, definition, types and significance
Definition
A molecular orbital (MO) is a three-dimensional region within a molecule where electrons are likely to be found. Molecular Orbitals are formed by the combination of atomic orbitals from different atoms through a process known as the linear combination of atomic orbitals (LCAO). These orbitals describe the distribution of electrons in a molecule, providing insights into its electronic structure, stability, and reactivity.
Introduction
In the complex world of chemistry, the behavior of atoms and molecules is governed by the fascinating realm of molecular orbitals. As electrons orbit nuclei, their interactions transcend individual atomic boundaries, giving rise to a collective electronic structure that defines the unique properties of compounds. Molecular Orbitals (MOs) provide a theoretical framework to understand this phenomenon.
What are molecular orbitals?
Molecular orbitals (MOs) are three-dimensional regions within a molecule where electrons are likely to be found. They arise from the overlap and interaction of atomic orbitals, dictating the distribution of electrons in a molecule.
Why are molecular orbitals important?
Understanding molecular orbitals is crucial for unraveling the electronic structure of molecules. They provide insights into bonding, stability, and reactivity, shaping our comprehension of the intricate world of chemistry.
Molecular orbital theory vs. valence bond theory Molecular Orbital Theory (MOT) and Valence Bond Theory (VBT) are two major approaches to understanding chemical bonding. While VBT emphasizes the overlap of atomic orbitals to form localized bonds, MOT considers the delocalization of electrons within molecular orbitals.
Linear combination of atomic orbitals (LCAO) method The LCAO method is a key concept in MOT, illustrating how atomic orbitals combine linearly to form molecular orbitals. This mathematical approach allows for the prediction of the molecular orbitals’ shapes and energies.
Types of Molecular Orbitals
Bonding molecular orbitals
Bonding molecular orbitals have lower energy and are more stable BMOs result from the constructive interference of atomic orbitals, leading to lower energy levels and increased stability. Electrons in these orbitals contribute to the formation of strong chemical bonds.
Antibonding molecular orbitals
Antibonding molecular orbitals have higher energy and are less stable ABMOs emerge from destructive interference, yielding higher energy levels and decreased stability. Electrons in ABMOs weaken bonds, making the molecule more reactive.
Non-bonding molecular orbitals
Non-bonding molecular orbitals have the same energy as parent atomic orbitals Non-bonding MOs, also known as lone pairs, retain the energy of the parent atomic orbitals. They contribute to the overall electronic structure of the molecule without participating directly in bonding.
Molecular Orbital Diagrams
How to construct a molecular orbital diagram Molecular orbital diagrams visually represent the arrangement of electrons in MOs. These diagrams follow the filling order of molecular orbitals based on the Aufbau principle, Pauli’s exclusion principle, and Hund’s rule.
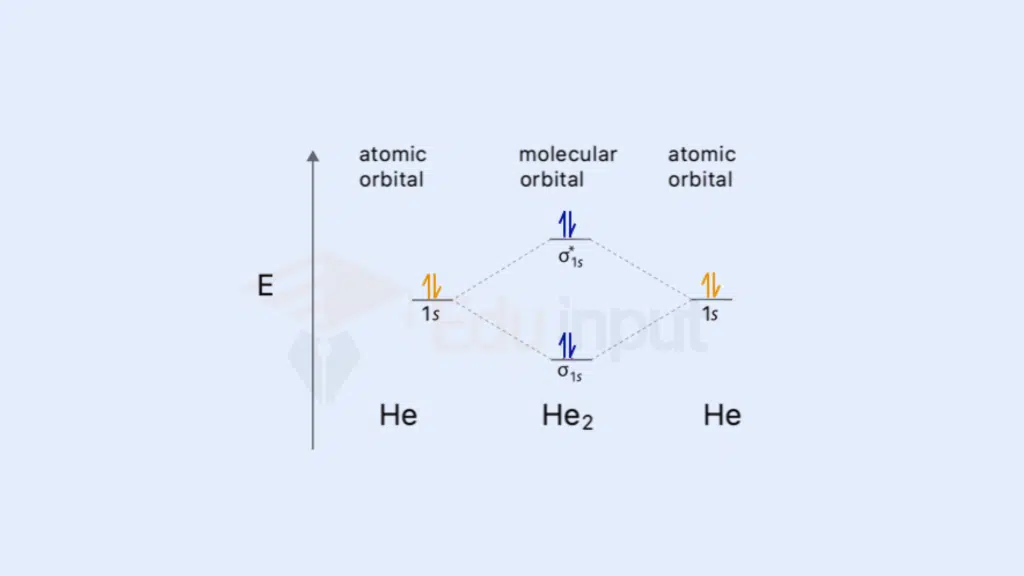
Examples of molecular orbital diagrams for simple molecules (e.g., H₂, He₂, O₂) For diatomic molecules like H₂, He₂, and O₂, molecular orbital diagrams illustrate the distribution of electrons in various orbitals, providing a foundation for understanding their stability and reactivity.
Bond Order and Bond Strength
Bond order
The number of bonding electrons minus the number of antibonding electrons Bond order quantifies the strength of a bond. It is calculated by subtracting the number of electrons in antibonding orbitals from those in bonding orbitals. Higher bond orders correspond to stronger and shorter bonds.
Bond strength
Increases with bond order As bond order increases, the strength of the bond also increases. This relationship is essential for predicting the stability and physical properties of molecules.
Significance of molecular orbitals
The significance of molecular orbitals (MOs) lies in their ability to provide a comprehensive understanding of the electronic structure, bonding, and properties of molecules. Here are some key aspects that highlight the significance of molecular orbitals:
Predicting Electronic Structure
Molecular orbitals allow scientists to predict the distribution of electrons within a molecule. This information is crucial for understanding the arrangement of electrons, their energy levels, and their influence on molecular properties.
Explaining Bonding in Molecules
MO theory provides a detailed explanation of how atomic orbitals combine to form molecular orbitals, elucidating the nature of bonding in molecules. It describes the constructive and destructive interference of atomic orbitals, leading to the formation of bonding and antibonding molecular orbitals.
Differentiating Bond Types
By analyzing the characteristics of molecular orbitals, one can distinguish between different types of chemical bonds, such as sigma (σ) bonds, pi (π) bonds, and others. MO theory provides insights into the strength, length, and stability of these bonds.






Leave a Reply