Exothermic Reactions-Characteristics, Identification, and Examples
Definition of Exothermic Reactions
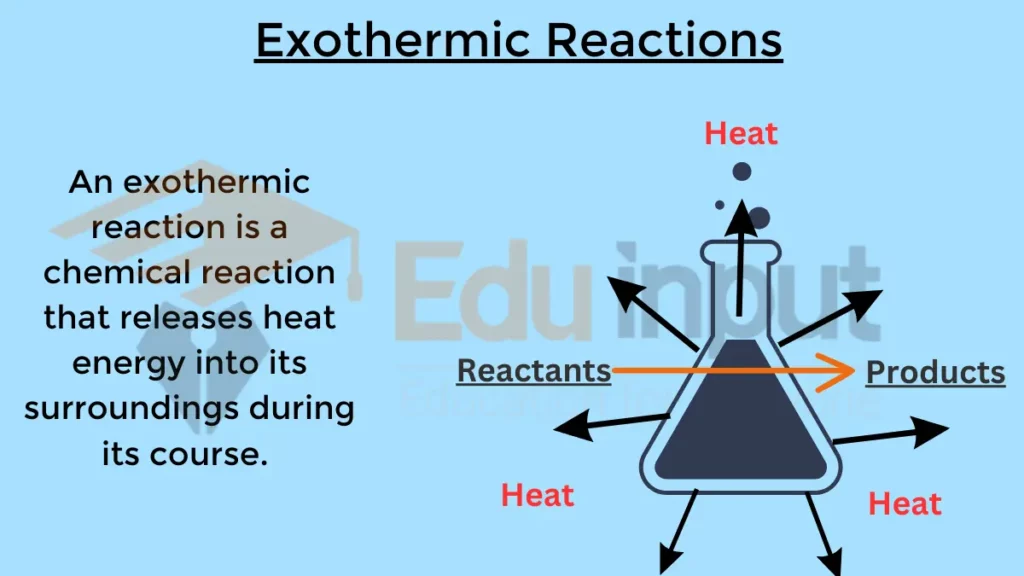
An exothermic reaction is a chemical reaction that releases heat energy into its surroundings during its course. Not all, but most of the spontaneous reactions are exothermic.
In simpler terms, these reactions produce more energy than they consume, resulting in an increase in temperature in the surrounding environment.
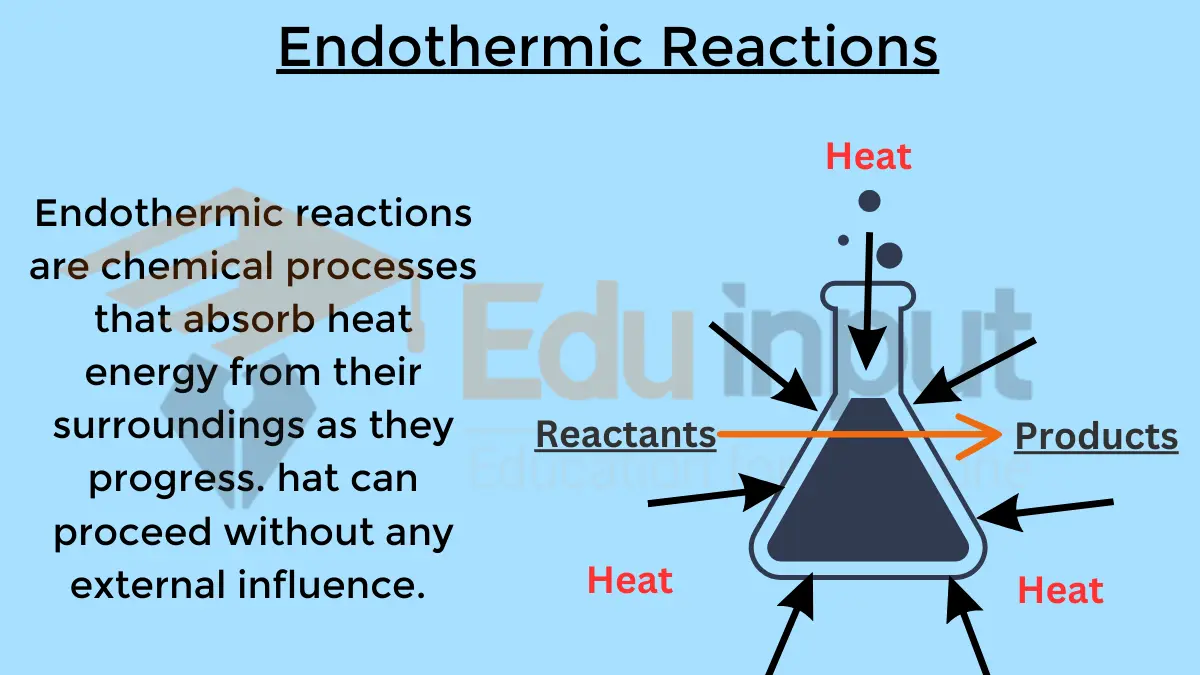
Also Read: Endothermic Reaction and Their Relation to Non-spontaneous reactions
Aalso read: Difference Between Endothermic And Exothermic Reactions
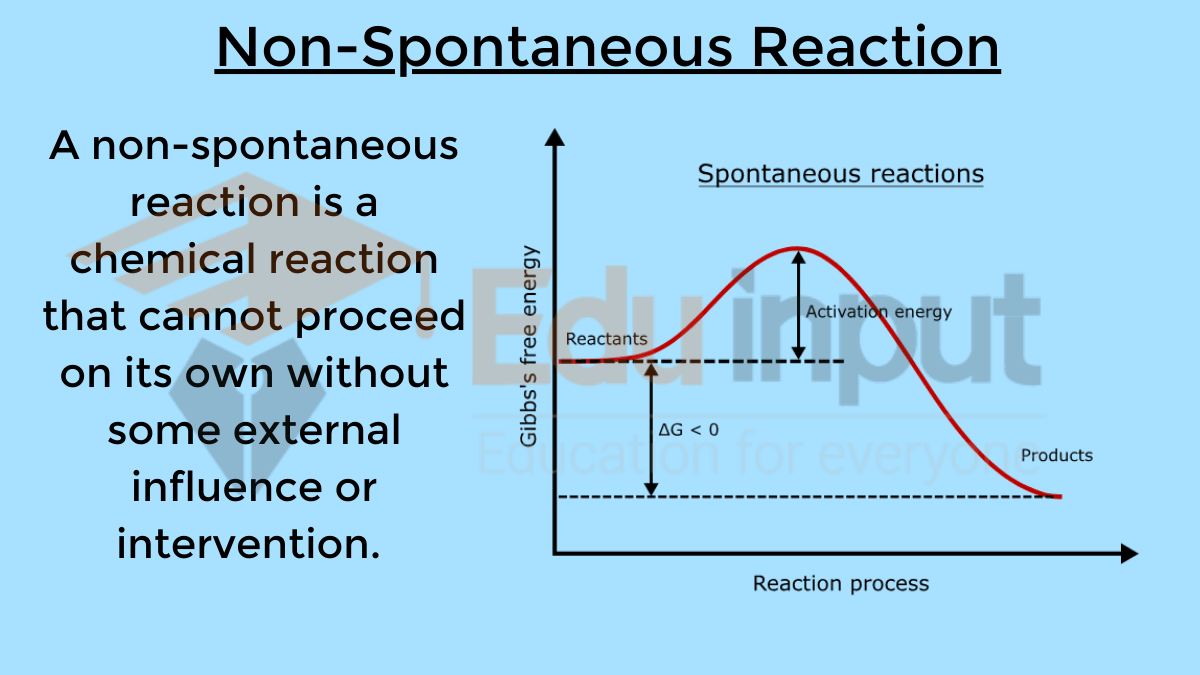
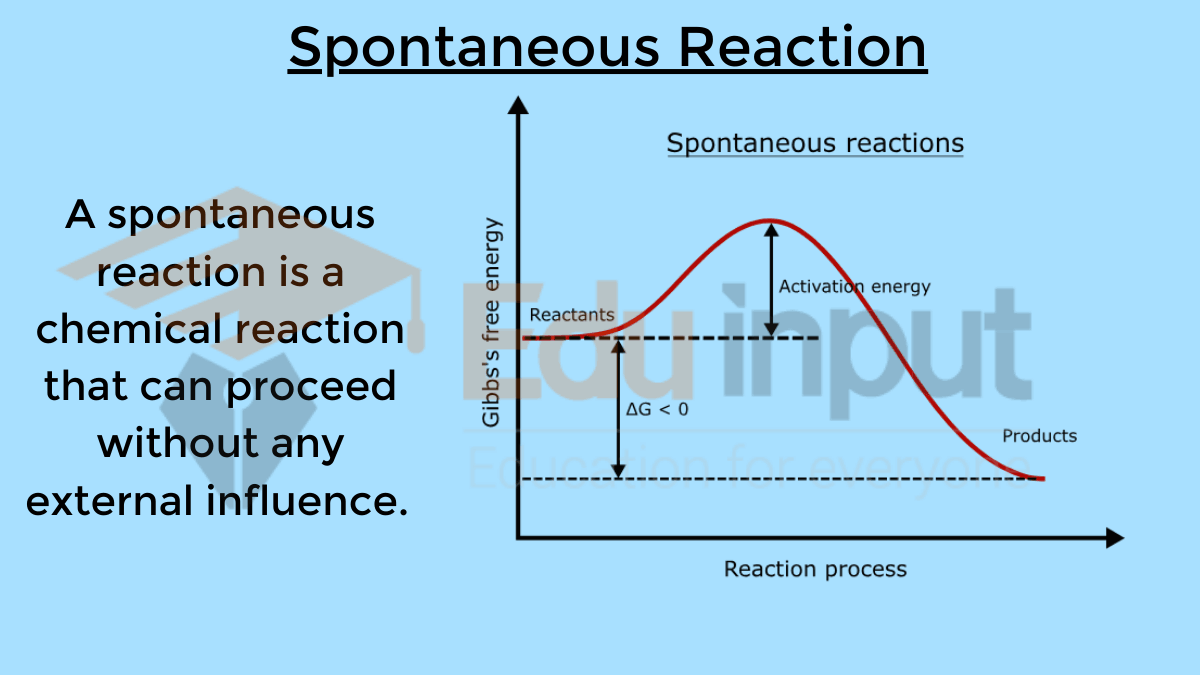
Difference between Spontaneous and Non-Spontaneous Reactions
Characteristics of Exothermic Reactions
Exothermic reactions exhibit several key characteristics –
1. Heat Release -The most defining characteristic is the release of heat energy into the surroundings during the reaction.
2. Temperature Increase -Exothermic reactions typically lead to an increase in temperature in the reaction mixture or surroundings.
3. Negative ΔH – The change in enthalpy (ΔH) for exothermic reactions is negative, indicating that the reaction releases energy.
4. Spontaneity – Exothermic reactions often occur spontaneously, as they involve a decrease in the overall energy of the system.
5. Heat as a Product – Heat is treated as a product in the balanced chemical equation of an exothermic reaction.
How Exothermic Reactions Occur?
Exothermic reactions occur as a result of the breaking and forming of chemical bonds. During a chemical reaction, bonds between atoms are broken in the reactants, and new bonds are formed in the products. This process involves the transfer of energy.
In exothermic reactions, the energy required to break the bonds in the reactants is less than the energy released when new bonds form in the products. The excess energy is released as heat into the surrounding environment. This release of heat is what characterizes exothermic reactions.
Why Exothermic Reactions Increase Temperature?
Exothermic reactions increase temperature because they release heat energy. This phenomenon can be explained by the law of conservation of energy, which states that energy cannot be created or destroyed; it can only change forms.
In the case of exothermic reactions, the potential energy stored in the chemical bonds of the reactants is converted into kinetic energy in the form of heat.
The increase in temperature occurs because the heat energy released by the reaction is transferred to the surrounding molecules, causing them to move faster and collide more frequently.
This increase in kinetic energy translates to a rise in temperature, making the environment warmer.
How to Identify Exothermic Reactions?
By observing the following signs, you can identify exothermic reactions and distinguish them from endothermic ones, which absorb heat from their surroundings –
Temperature Rise – If you observe a significant increase in temperature during a chemical reaction, it’s likely exothermic. Use a thermometer or feel the container for warmth.
Heat Sensation – You may feel the container or reactants getting hot to the touch, indicating heat release.
Flame or Light – Some exothermic reactions produce flames or light, like combustion reactions.
Gas Release – Look for the production of gases, often accompanied by heat. Bubbling or expansion is a common sign.
Color Change – Exothermic reactions can cause noticeable color changes in substances involved.
Steam or Odor – Steam or a distinct odor may be emitted during the reaction.
Thermochromic Compounds – Some substances change color with temperature changes.
Thermochromic Indicators – In controlled experiments, use thermochromic indicators to visually confirm temperature changes associated with heat release.
Examples
Exothermic reactions include the combustion of fuel, rust formation, explosions in fireworks, hand warmers generating heat, cooking on a stove, igniting matches, neutralization reactions, natural processes like volcanic eruptions, thermal decomposition of substances, and exothermic biochemical reactions in digestion.



Leave a Reply