Non-Spontaneous Reactions-Thermochemistry, and Examples
A non-spontaneous reaction is a chemical reaction that cannot proceed on its own without some external influence or intervention.
Like spontaneous reactions, non-spontaneous reactions are determined by the enthalpy and entropy changes during the reaction.
But in non-spontaneous reactions, these thermodynamic factors sum to an unfavorable Gibbs free energy change that prevents the reaction from proceeding unaided.
Also read: Difference between Spontaneous and Non-Spontaneous Reactions
Introduction to Thermochemistry
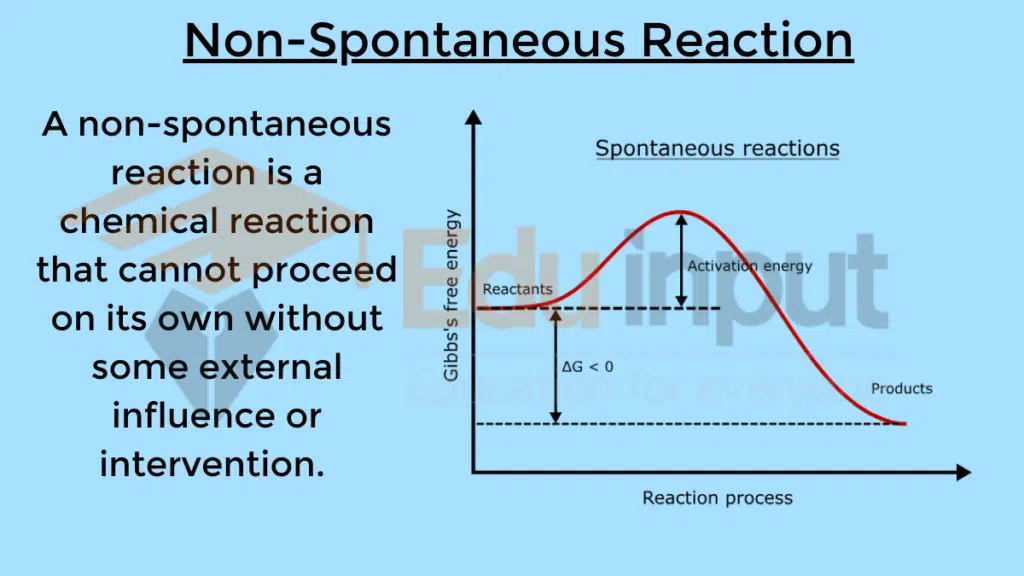
Thermodynamics of Non-Spontaneous Reactions
For a reaction to be non-spontaneous, the Gibbs free energy change (ΔG) must be positive under the conditions of interest.
ΔG = ΔH – TΔS > 0
A positive ΔG means the reaction as written will not take place spontaneously. Either the enthalpy term dominates so that ΔH is highly positive, or the entropy term TΔS is negative enough to make ΔG positive overall.
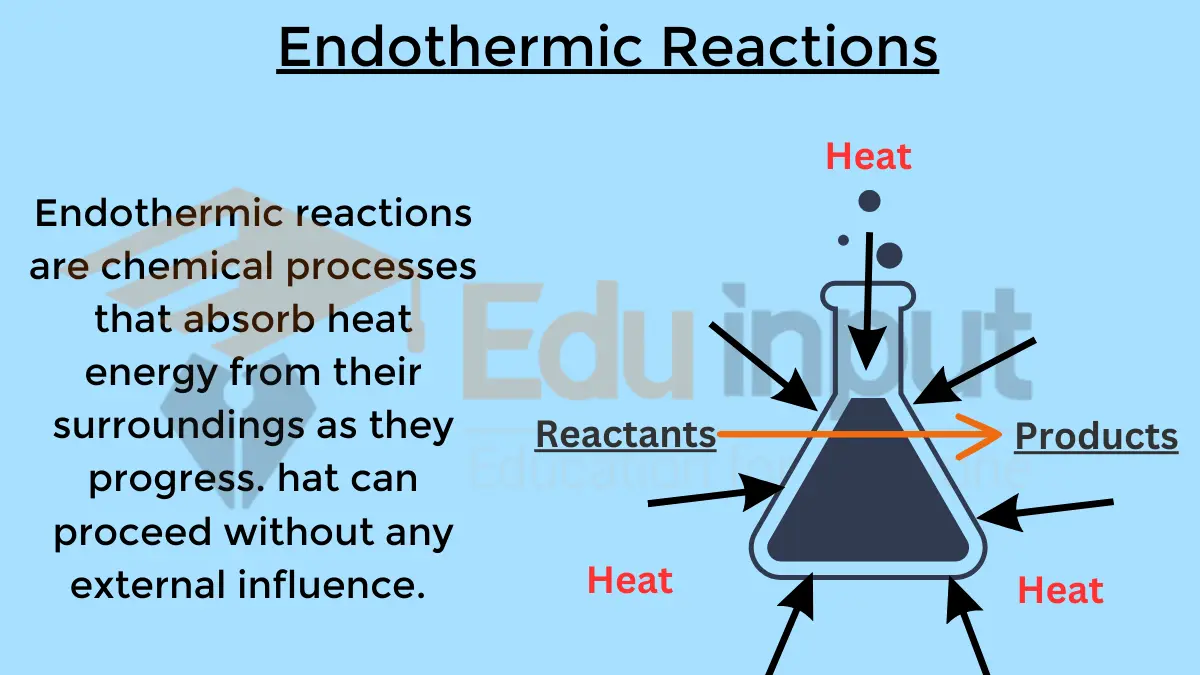
In endothermic reactions where heat is absorbed, ΔH is positive. If the entropy change is also unfavorable, the reaction will be non-spontaneous. Reactions that involve complex molecule or ordered crystal formation have negative entropy changes.
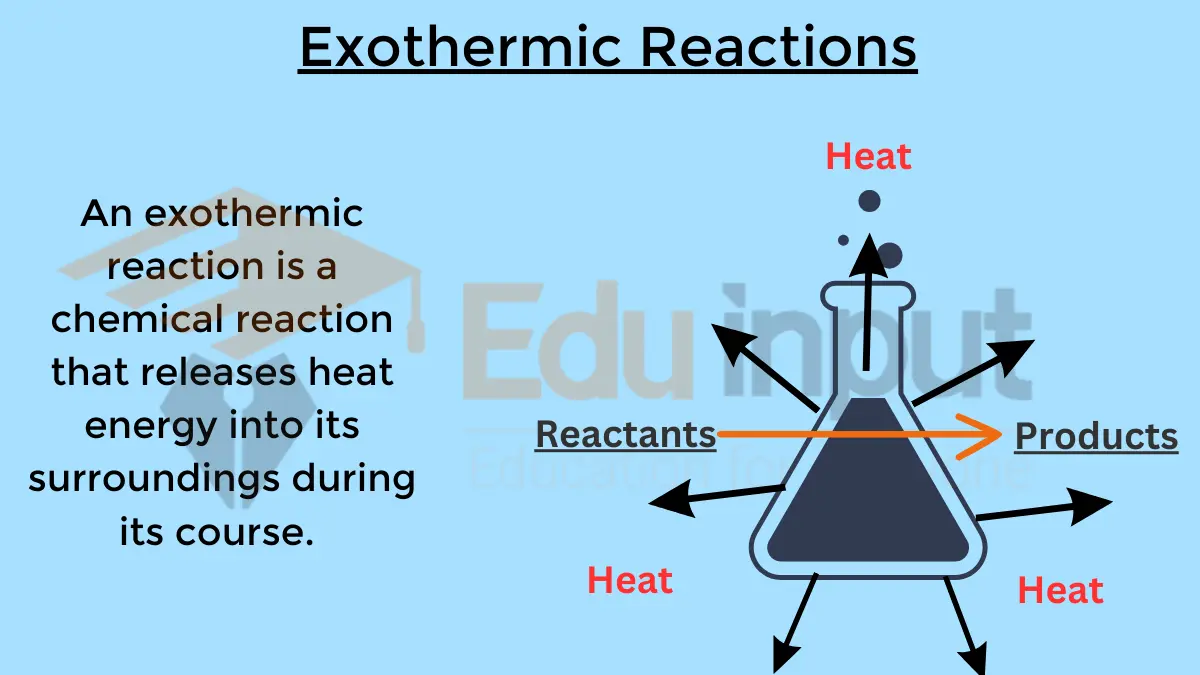
You might be interest to learn about Exothermic Reactions
Gibbs Free Energy and Non-Spontaneity
The Gibbs free energy directly reflects whether or not a chemical reaction will occur spontaneously.
For non-spontaneous reactions:
ΔG > 0
The more positive the ΔG, the more non-spontaneous the reaction is. A large positive ΔG means the reaction is extremely uphill and energetically unfavorable in the direction written.
ΔG lets us predict the theoretical extent to which a non-spontaneous reaction can be driven in the forward direction to produce a desired product.
Reversibility of Non-Spontaneous Reactions
Non-spontaneous reactions can be either reversible or irreversible.
Reversibility depends on the kinetics of the forward and reverse reactions, not on spontaneity. A non-spontaneous reaction is at equilibrium when the forward and reverse rates are equal, making it reversible.
For example:
A + B ⇌ C + D
If this reaction is non-spontaneous in the forward direction (positive ΔG), it will reach an equilibrium state where detectable amounts of both reactants and products are present. This is a reversible non-spontaneous reaction.
However, some non-spontaneous reactions may be irreversible in practice. This can happen for kinetic reasons if the activation energy for the reverse reaction is much higher than for the forward reaction. In this case, the products do not convert back into reactants once formed.
An example is ATP hydrolysis:
ATP + H2O → ADP + Pi
This is a non-spontaneous reaction in the forward direction, requiring energy input for ATP synthesis. However, the hydrolysis is essentially irreversible under cellular conditions due to its fast kinetics.
Chemical Kinetics of Non-Spontaneous Reactions
Chemical kinetics of Non-Spontaneous Reactions is the study of the rate of chemical reactions. The rate of a reaction is the speed at which the reactants are converted into products.
The rate of a chemical reaction is affected by a number of factors, including:
- The concentration of the reactants
- The temperature
- The presence of a catalyst
The rate of a non-spontaneous reaction is typically slower than the rate of a spontaneous reaction. This is because non-spontaneous reactions require the input of energy. This energy can be provided in a number of ways, such as by heating the reaction mixture or by adding a catalyst.
Examples of Non-Spontaneous Reactions
Many important reactions in nature and technology do not occur spontaneously:
- Nitrogen fixation – Converting N2 to NH3 for fertilizers requires enormous energy input and catalysts.
- Photosynthesis – CO2 and H2O converging to glucose and O2 only occurs in plants with sunlight energy.
- Phosphorylation – ATP synthesis from ADP + Pi in cells requires actively pumping protons across membranes.
- Electroplating – Depositing metal coatings like chromium or gold needs an external electrical voltage applied.
Driving Non-Spontaneous Reactions
Non-spontaneous reactions need an extra energy boost. there are the following ways to drive Non-Spontaneous Reactions :
- Increase temperature – Heating shifts the equilibrium towards the endothermic direction per Le Chatelier’s principle.
- Add a catalyst – Catalysts lower activation energy but do not alter thermodynamic favorability.
- Couple to a spontaneous process – Using a spontaneous reaction’s Gibbs free energy can drive a non-spontaneous reaction. Fuel cells and ATP synthesis exemplify this.
- Apply external energy – Direct forms of energy input like light, voltage, ultrasound, etc. can be used to force non-spontaneous reactions to occur.
Aalso read: Difference Between Endothermic And Exothermic Reactions



Leave a Reply