Spontaneous Reactions-Thermochemistry, and Examples
A spontaneous reaction is a chemical reaction that can proceed without any external influence. In other words, it can occur naturally once the reactants are present.
In thermochemistry of Spontaneous Reactions, Enthalpy, or heat content, provides information about the energy flow during a reaction. Entropy measures the dispersal of energy and matter.
By considering both enthalpy and entropy changes, we can predict whether or not a reaction will proceed spontaneously.
Also read: What are Non-Sponteneous Reactions?
Difference between Spontaneous and Non-Spontaneous Reactions
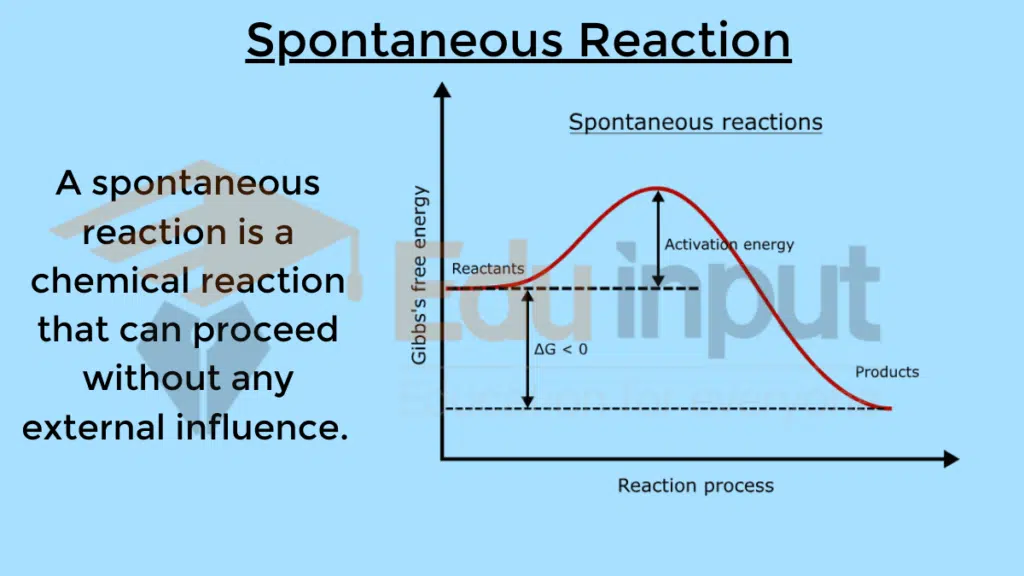
Characteristics of Spontaneous Reactions
Key characteristics of spontaneous reactions are:
- Negative Gibbs free energy change (ΔG < 0)
- Proceeds without external influence
- Irreversible under reaction conditions
- Exothermic nature (releases heat)
- Increased randomness (positive ΔS)
- Rapid reaction rate
- Difficult to control or stop
- Occur under standard conditions
- Give off signs like heat, gas bubbles, color change
Thermochemistry of Spontaneous Reactions
The enthalpy change (ΔH) of a reaction indicates whether the reaction absorbs or releases heat. Exothermic reactions that release heat have a negative ΔH, while endothermic reactions that absorb heat have a positive ΔH.
Entropy change (ΔS) quantifies the dispersal of energy and matter. Reactions that produce more disorder or randomness have a positive ΔS. Well-ordered crystalline solid formation has a negative ΔS.
For a process to occur spontaneously, the sum of enthalpy and entropy factors must be favorable. This is expressed through Gibbs free energy (ΔG), where ΔG = ΔH – TΔS. A negative ΔG indicates a spontaneous reaction.
If ΔH is negative and ΔS positive, ΔG will always be negative and the reaction spontaneous. But spontaneity also depends on temperature and relative contribution of enthalpy vs. entropy.
The value of δG at which a reaction becomes spontaneous is negative. This is because the Gibbs free energy change (δG) is a measure of the spontaneity of a reaction, and a negative δG indicates that the reaction will proceed spontaneously.
Gibbs Free Energy and Spontaneity
The Gibbs free energy function allows us to predict whether a reaction will be spontaneous under defined temperature and pressure conditions.
For a spontaneous reaction:
ΔG = ΔH – TΔS < 0
If ΔG is negative, the reaction will proceed spontaneously as written. A positive ΔG means the reaction is non-spontaneous.
The more negative ΔG, the more favorable the spontaneity. ΔG lets us determine the theoretical yield of a reaction by finding the extent to which it can progress spontaneously.
Irreversible and reversible reactions
Spontaneous reactions can be either irreversible or reversible. Irreversible reactions proceed to completion in one direction, while reversible reactions can proceed in either direction.
Examples of irreversible spontaneous reactions include:
- Combustion reactions
- Acid-base reactions
- Precipitation reactions
Examples of reversible spontaneous reactions include:
- The Haber process (synthesis of ammonia)
- The Ostwald process (synthesis of nitric acid)
- The fermentation of glucose to ethanol
Chemical Kinetics of Spontaneous Reactions
Kinetics is the study of the rate of chemical reactions. Even if a reaction is spontaneous, it may not occur quickly.
The rate of a reaction depends on a number of factors, including:
- The concentration of the reactants
- The temperature
- The presence of a catalyst
What makes a chemical reaction spontaneous?
A chemical reaction is spontaneous if it can occur without any external input of energy. The spontaneity of a reaction is determined by the Gibbs free energy change (ΔG).
ΔG is calculated using the following equation:
ΔG = ΔH - TΔS
where:
- ΔG is the change in Gibbs free energy
- ΔH is the change in enthalpy
- T is the temperature in Kelvin
- ΔS is the change in entropy
Enthalpy (ΔH) is the change in energy during a chemical reaction. An exothermic reaction releases energy, while an endothermic reaction absorbs energy.
Entropy (ΔS) is the measure of disorder in a system. A spontaneous reaction is one that increases the disorder of the universe.
A reaction is spontaneous if ΔG is negative. This means that the reaction releases more energy than it absorbs and/or increases the disorder of the universe.
Examples of Spontaneous Reactions
Many reactions occur spontaneously around us, like:
- Ripening of fruits – Complex sugars break down into simpler sugars, flavor compounds, and CO2.
- Burning of wood – Combustion of cellulose and lignin is an exothermic redox reaction.
- Curdling of milk – Proteins denature and aggregate spontaneously in acidic conditions.
- Battery discharge – Oxidation of metals and reduction of oxygen occurs spontaneously, releasing energy.
Also read: Difference Between Endothermic And Exothermic Reactions



Leave a Reply