What is Coulomb’s Law? | A Detailed Introduction
Coulomb’s law is applicable to electric charges. There are two types of Electric charges positive and negative. The same charges repel each other. For example, if we have 2 negative or 2 positive charges then they will repel each other.
Opposite charges attract each other. It means if we have one positive and one negative charge or then there will be a force of attraction between them.
This is the qualitative analysis of force between charges which tells us that there are two types of force between charges.
Force of Attraction
Force of Repulsion
Download the pdf notes of coulomb’s law Class 12 Physics
Coulomb’s law
In 1786 AD Charles coulomb who was a French military engineer gave coulomb’s law. This gives the quantitative analysis of force (attraction or repulsion). It is an empirical law and he deduces this law from many experiments.
When coulomb’s law is applicable
Coulomb’s law is only applicable when
- Charges should be static
- Charges must be point charges
- Charges are far enough so that strong nuclear force does not dominate over coulomb force
What is a point charge?
A charge is said to be a point charge when the distance between charges is far greater than their magnitude.
We know that the same charges repel each other but in an atom, there are protons in the nucleus and they have a positive charge on them.
They should repel each other as they have the same positive charge but they attract each other because when we bring the same charges very close to each other a strong nuclear force of attraction is produced between them. This force is very strong and dominates the force of repulsion. Coulomb’s law does not hold in this condition.
If we want to apply the coulomb’s law on charges then there should be not any nuclear force between them.
Definition of coulomb’s law
The force of attraction or repulsion between two static charges is directly proportional to the product of magnitudes of charges and inversely proportional to the square of the distance between them.
Mathematical derivation of coulomb’s law
We suppose that we have two charges of magnitude q1 and q2. And F is the force between them then according to coulomb’s law.
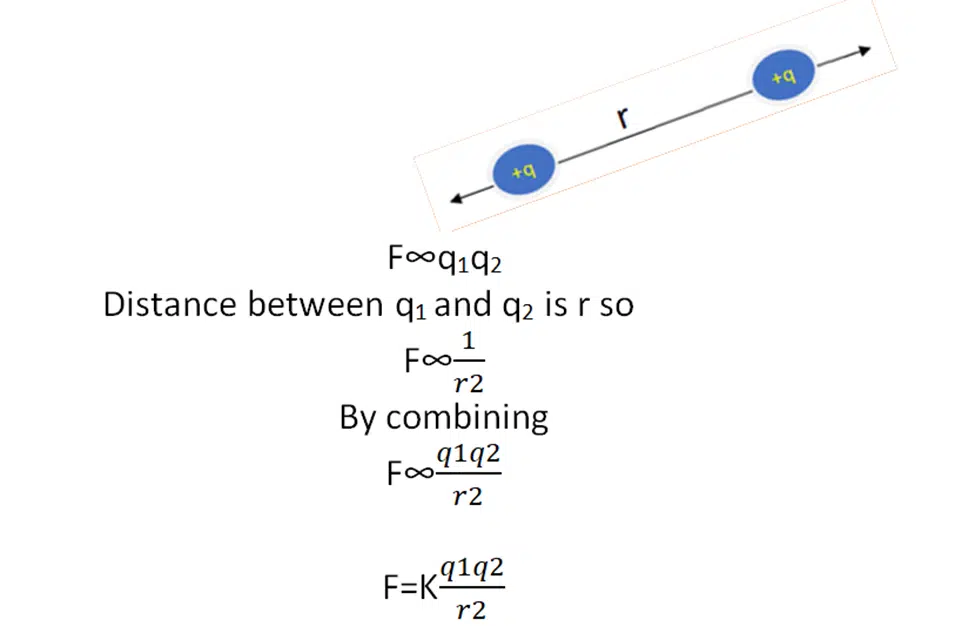
K is the proportionality constant and is known as the coulomb’s constant.
It is not a universal constant. Its values depend on
- Nature of medium between charges
- System of units
Unit of K
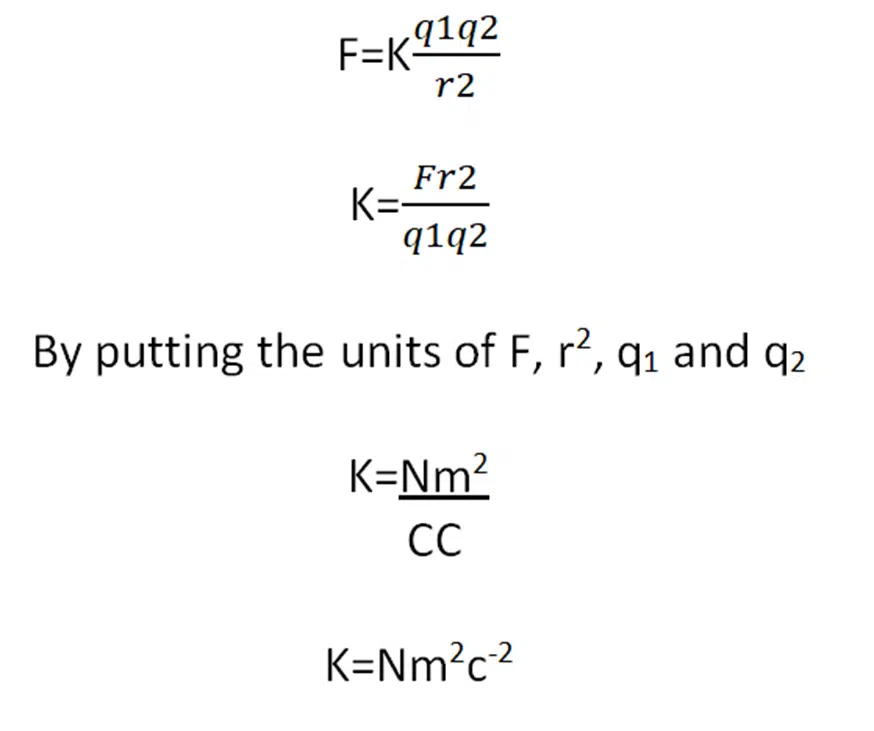
Coulomb’s law in free space or vacuum
The value of K is not fixed. The value of K is different in different mediums. The value of K for free space is
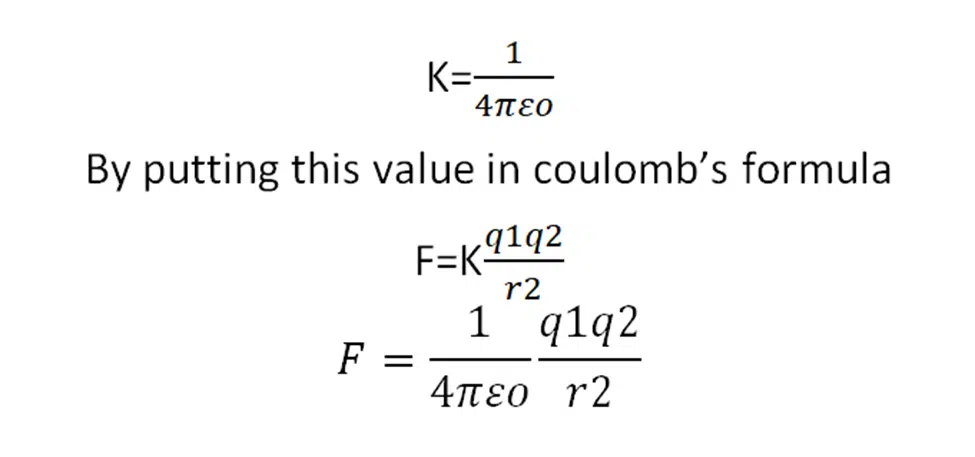
This is the coulomb’s force in vacuum or free space and εo is the permittivity of free space.
What is Permittivity?
Permittivity is taken from the word permeation. It means how much permeation is given by the medium to force it to pass through it. But actually, permittivity means
How much does medium allow its own field to reduce the coulomb’s forces
Coulomb’s force and permittivity have opposite relations.
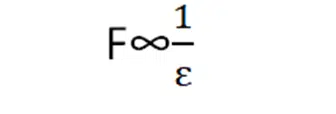
If the medium has more permittivity it will allow the coulomb’s force to pass through it less. The value of permittivity for free space is 8.85×10-12N-1m-2C2. The value of K in free space is

The permittivity of free space is minimum so the maximum coulomb’s force will pass through it.
If the permittivity of a medium is maximum then the coulomb’s force will be minimum.
Coulomb’s force in the medium
Coulomb’s force depends on the permittivity of the medium and it varies with the medium between charges. If we want to find the coulomb’s force in a particular medium then we compare the permittivity of that medium to the permittivity of space.
What is Relative permittivity?
The ratio between the permittivity of medium and space is called relative permittivity.
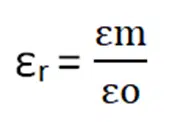
Suppose the permittivity of the medium is εm then the force in that medium will be
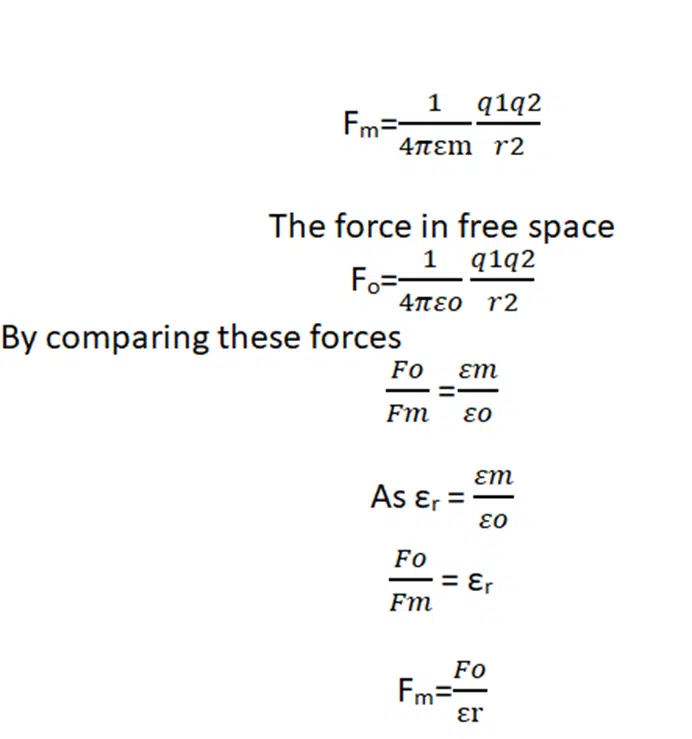
It means that force in the medium is 1/εt times the force in free space.
Since the permittivity of free space is minimum as compared to any medium so the force in free space will be maximum in free space than any other medium.
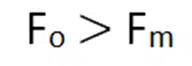
Vector form of coulomb’s law
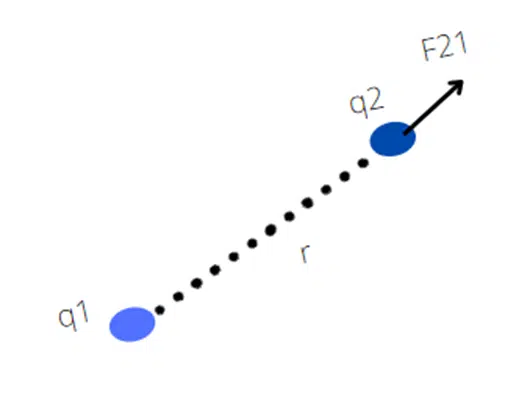
Coulomb’s force is mutual force. According to Newton’s third law when charge q1 exerts a force on q2 then at the same time q2 also exerts a force on q1 which is equal in magnitude but opposite in direction. This force act along the line joining the center of charges.
If the F21 is a force on q2 by q1
F12 is a force on q1 by q2
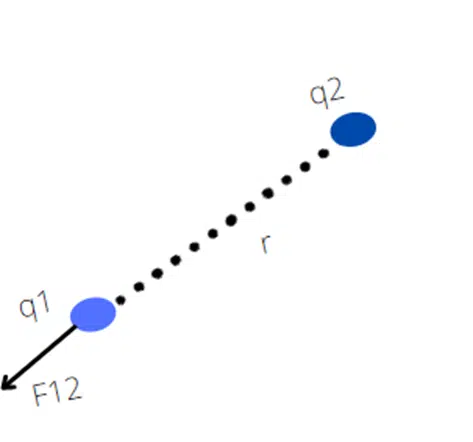
Then according to Newton’s law
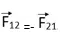
Force is a vector quantity. We multiply the unit vector with magnitude to find the vector quantity.
ȓ21 is the unit vector of F21
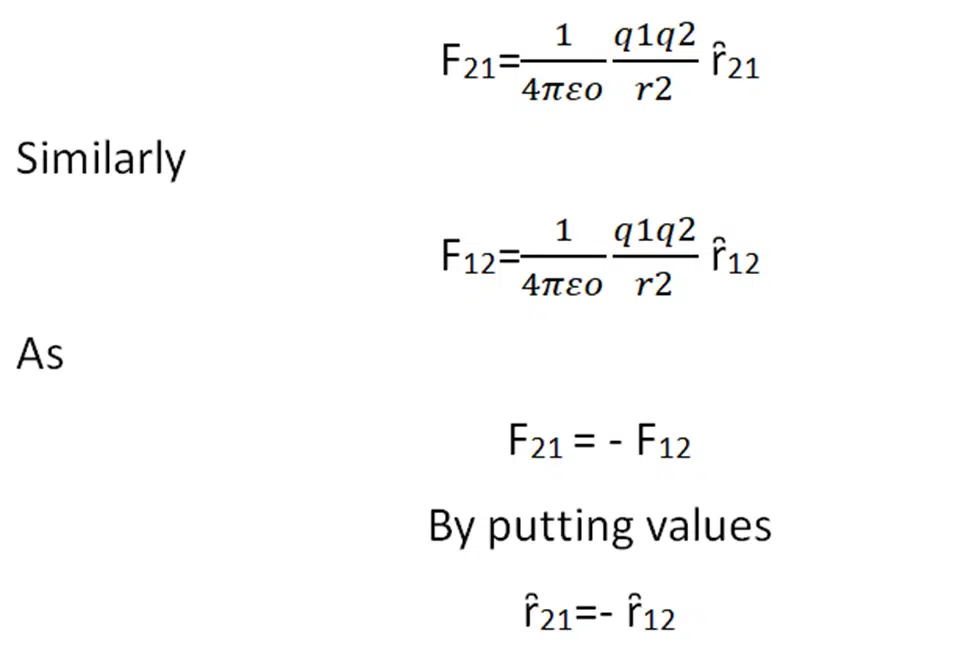
It means the unit vectors are also the same in magnitude but opposite in direction. Now we solve some numerical related to coulomb’s law.
Coulomb’s Law: Problems and Solutions
- Compute the electric force between two charges of 5×10−9 C and −3×10−8 C which are separated by
d = 10cm.
Solution:
The magnitude of the electrostatic force between two point charges is given by Coulomb’s law as
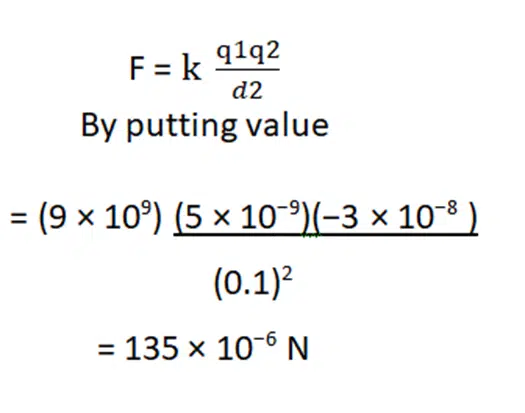
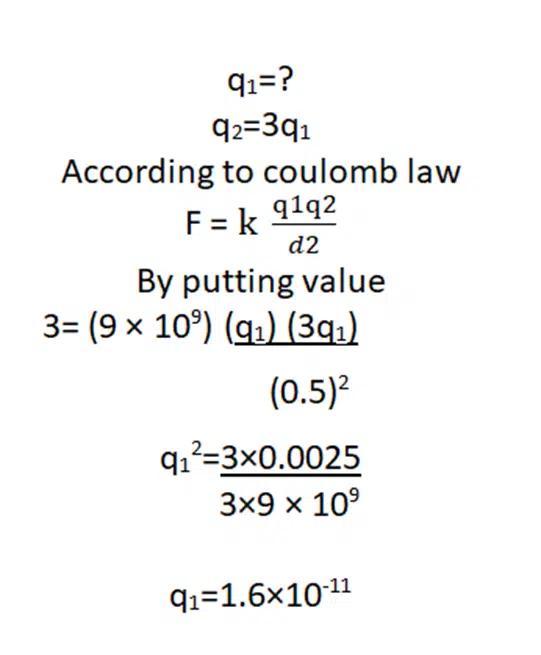
2: Two spheres located at a distance of d = 5 cm attract one another with a force of F = 3 N. If one of them has three times more charges than the other, find the electric force between them.
Solution:





Leave a Reply