Nuclear Reaction | Rutherford Experiment on Nuclear Reaction
A nuclear reaction is a process in which two nuclei, or a nucleus, collide to produce one or more new nuclides. The nuclear reaction also takes place in Nuclear reactors to produce an electric current. It is an irreversible reaction.
Types of Nuclear Reaction
These are 4 types of nuclear reaction
- Nuclear fission reaction
- Fission Chain Reaction
- Nuclear Decay reaction
- Nuclear Fusion reaction
- Nuclear Transmutation
Nuclear Reaction Formula
While studying radioactivity, we have seen that a particle is emitted from radium-226, and radon-222 is obtained. This nuclear change is represented by the following equation

Such an equation represents a nuclear reaction. The above-mentioned nuclear reaction takes place of its own accord.
However, it was Rutherford who, first of all, expressed his opinion that besides natural radioactive decay processes, other nuclear reactions can also occur.
A particle x is bombarded on any nucleus X and this process yields a nucleus Y and a light object y as

Rutherford Experiment on Nuclear Reaction
Rutherford performed an experiment on nuclear reactions in 1918. He bombarded α-particles with nitrogen. He observed that as a result of this reaction, oxygen is obtained and a photon is emitted.
This reaction indicated that when the α-particle enters the nucleus of 147N then an excitation is produced in it. And as a result of it, 178O and a proton are produced.

Since experiment Rutherford, countless nuclear reactions have been observed. For nuclear reactions to take place the fulfillment of certain conditions is a must.
Condition for Nuclear Reaction
Before and after any nuclear reaction the number of protons and neutrons must remain the same because protons and neutrons can neither be destroyed nor can they be created.
Let’s elaborate on this point from the example of Rutherford’s nuclear reaction of

Number of protons=7+2=8+1
Number of neutrons=7+2=9+0
A nuclear reaction can take place only when the total energy of the reactants including the rest mass energy is equal to the total energy of the products.
In this reaction the mass of the reactants is
Mass of 147N = 14.0031 u
Mass of 42He = 4.0026 u
The total mass of the reactants = 18.0057 u
In the same way, the mass of the products is
Mass of 178O = 16.9991 u
Mass of 11H =1.0078u
The total mass of the products after the reaction = 18.0069 u
This shows that the total mass after the reaction is greater than the total mass before the reaction by 0.0012 u.
1u mass=931 MeV energy, therefore a mass difference of 0.0012u is equivalent to an energy of 931 MeV x 0.0012 u= 1.13 MeV.
Hence this reaction is possible only when an additional mass of 0.0012 u is added to the reactants or the minimum kinetic energy of the α-particle is 1.13 MeV such as obtained from 2144Po.
The energy of these α-particles is equal to 7.7 MeV which is more significant than 1.13 MeV. Hence these α-particles were obtained from a source that gives out α-particles whose energy was less than 1.13 MeV then this reaction would not have taken place.
An interesting aspect of a nuclear reaction is that it can also take place in the opposite direction.
Nuclear Reaction Example
178O is obtained by the interaction 147N with an α-particle of appropriate energy. If we accelerate protons, with the help of a machine like a cyclotron, increase their velocity and then bombard these high-velocity protons on 178O, Rutherford’s nuclear reaction of 147N and α-particle will proceed in the backward direction as

By bombarding different elements with α-particles, protons, and neutrons, many nuclear reactions have been produced.
Discovery of Neutron
One such nuclear reaction with the help of which James Chadwick discovered the neutron in 1932.
When Be was bombarded with α-particles emitted out of 21084Po, then as a result of a nuclear reaction, 126C and a neutron were obtained.

As neutron carries no charge, therefore, it presented a great amount of difficulty for its identification. Anyhow when neutrons were passed through a block of paraffin, fast-moving protons were ejected out and these were easily identified.
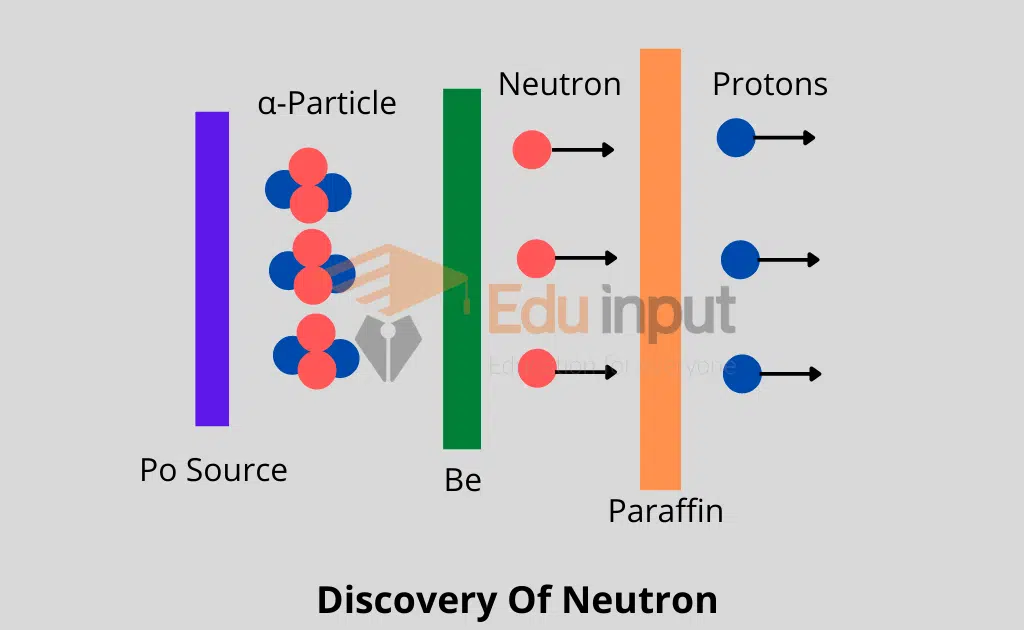
It may be remembered that a large amount of hydrogen is present in paraffin and the nuclei of hydrogen atoms are protons.
The emission of protons is the consequence of elastic collisions between the neutrons and the protons. This indicates that the mass of a neutron is equal to the mass of the proton.
It may be remembered that when an object of a certain mass collides with another object of equal mass at rest, then as a result of the elastic collision, the moving object comes to rest and the stationary object begins to move with the velocity of the colliding object.
The discovery of neutrons has brought a revolution in nuclear reactions, as the neutrons carry no charge so they can easily enter the nucleus.
Frequently Asked Question
What is the nuclear reaction?
A nuclear reaction is a process in which two nuclei, or a nucleus, collide to produce one or more new nuclides.
How many types of nuclear reactions?
There are 4 types of nuclear reaction
· Nuclear fission reaction
· Nuclear Decay
· Nuclear Fusion reaction
. Nuclear Transmutation
What is the nuclear equation?
Nuclear equations represent the reactants and products in nuclear fission or nuclear fusion.
Who discovered the neutron?
James Chadwick discovered the neutron in 1932.
When does the nuclear reaction take place?
A nuclear reaction can take place only when the total energy of the reactants including the rest mass energy is equal to the total energy of the products.





Leave a Reply