Solvent Extraction -Types, Principle, uses
What is solvent extraction?
It is a technique in which a solute can be separated from a solution by shaking the solution with a solvent in which the solute is more soluble and the added solvent does not mix with the solution.
solvent extraction Definition

There are different types of solvent extraction, that are based on phases of extraction, Solvent State, Energy Source, Operation Mode, and Contact Mode. Many other factors are also involved in it.
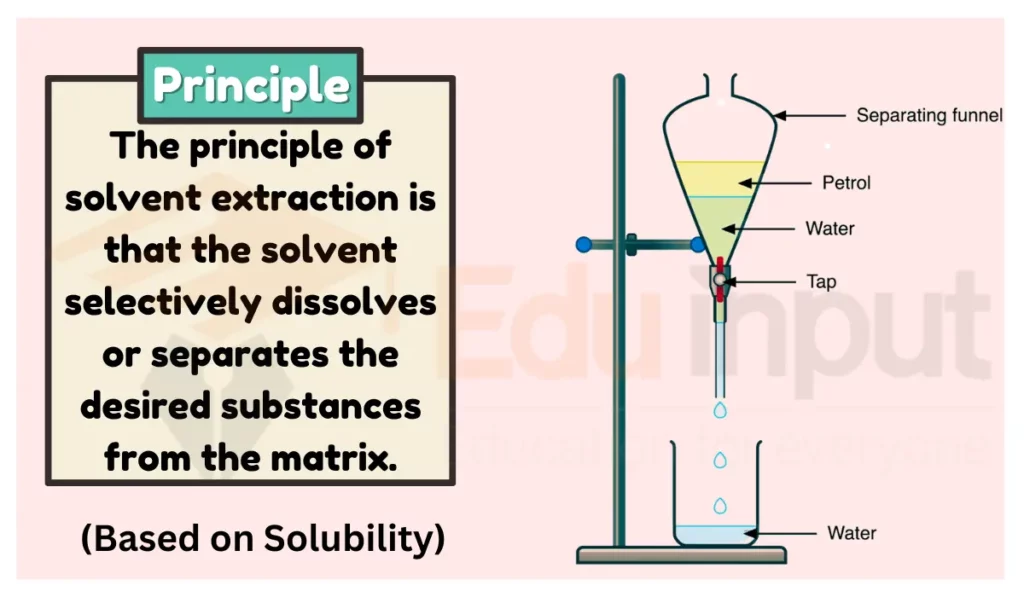
Principle of Solvent Extraction
The principle of solvent extraction is based on the fact that different substances have different solubility in different solvents. In solvent extraction, a solvent is used to extract a desired substance from a mixture. The solvent is chosen in such a way that the desired substance is soluble in it, while the other substances in the mixture are not. This allows the desired substance to be separated from the mixture.
Read Which Properties of Solvents are Useful for Solvent Extraction
Read: Which Factors Affect Process of Solvent Extraction
Characteristics of solvent extraction
The main characteristics of solvent extraction are as follows
- It is an equilibrium process and follows the distribution law.
- This technique is particularly useful when the product is volatile or thermally unstable.
- It is a convenient technique and is carried out in a separating funnel.
Distribution Law or Partition Law
This law states that the ratio of a solute distributes itself between two immiscible liquids in a constant ratio of concentrations irrespective of the amount of solute added.
Distribution Co-efficient
The ratio of the concentration of solute dissolved in two immiscible liquids at equilibrium is called the distribution coefficient.
K = concentration of solute in organic phase/ concentration of solute in the aqueous phase
Explanation
Solvent extraction is a process used in chemistry to separate and isolate a specific compound or group of compounds from a complex mixture. The technique involves using a solvent to selectively dissolve the target compound(s) from the mixture, allowing them to be separated from other components.

In the process of solvent extraction, the solution containing the mixture is typically placed in a separating funnel along with a second liquid, which serves as the solvent. The separating funnel is a conical-shaped glass vessel with a stopcock at the bottom, which allows for the controlled release of the liquids.
Once the two liquids are added to the separating funnel, the funnel is stopped and the contents are shaken together. This allows for the mixing of the two liquids, which facilitates the transfer of the target compound(s) from the original solution to the solvent. The two liquids are then allowed to separate into two layers, with the denser layer (typically the solvent layer) settling at the bottom of the funnel and the lighter layer (typically the solution layer) floating on top.
Finally, the stopcock is opened and the denser solvent layer is carefully drained into a separate container, leaving the target compound(s) behind in the original solution layer. The solvent layer can then be further processed to isolate and purify the target compound(s) as needed.
Overall, we can say solvent extraction is governed by which distribution law. Solvent extraction is a powerful and versatile technique widely used in chemical analysis and other fields such as pharmaceuticals, food science, and environmental science.
Read How Solvent extraction is different from distillation?
Solvent Extraction Method Examples
Ether Extraction or Liquid-liquid extraction
Ether extraction is one of the most commonly used methods to understand the process of solvent extraction. It is also known as liquid-liquid extraction because water and ether are used as a liquid.
The most common laboratory example of solvent extraction is ether extraction. This is used to separate products of organic synthesis from water.
In a typical organic synthesis, the aqueous solution containing the organic product is shaken up with ether in a separating funnel and allowed to separate.
The organic impurities remain in the aqueous phase whereas the compound goes to the ether layer.
The ether layer is separated and the organic compound is obtained by evaporating the ether.

Efficiency of the process
Repeated extraction by using a small portion of the solvent is more efficient than using a single but large volume of solvent.
Applications of Solvent Extraction
Some common uses of solvent extraction:
- In pharmaceutical industry: In pharmaceutical industry, solvent extraction is used to extract active ingredients from plants and other natural sources. It is also used to separate and purify different components of a drug.
- In petrochemical industry: In the petrochemical industry, solvent extraction is used to separate and purify different components of crude oil. It is also used to extract chemicals from waste streams.
- In food processing industry: In the food processing industry, solvent extraction is used to extract flavors and fragrances from natural sources such as fruits and flowers. It is also used to extract oils from seeds and nuts.
- Removing caffeine from coffee beans – After the beans are soaked in water, a solvent like dichloromethane is used to pull the caffeine out from the water. This allows decaffeinated coffee to be made.
- Getting vegetable oils from seeds and nuts – Hexane solvent pulls the oils out from inside seeds like soybeans. The oils are then purified and used for cooking.
- Extracting flavors and fragrances – Ethanol solvent draws out the aromatic flavors and scents from plants. These are used to make perfumes, food flavorings, etc (Or read our detailed article on why is ethanol a good solvent for solvent extraction?)
- Cleaning up oil spills – Solvents break down and remove oil and grease that has spilled into water. This helps clean up environmental pollution.
- Purifying metals – Solvents dissolve metals like copper and gold out of crushed ore rocks. The pure recovered metals are then used to make wires, jewelry, and more.
Advantages and Disadvantages of Solvent Extraction
The advantages of solvent extraction include its ability to extract a wide range of substances, its simplicity, and its cost-effectiveness. However, there are also some disadvantages to this technique. One of the major disadvantages is that it can be harmful to the environment if not properly disposed of. Additionally, the use of solvents can result in the loss of some of the desired substance.
Faqs
Why is solvent extraction used?
Solvent extraction is used to separate and isolate different substances from a mixture. It is commonly used to remove harmful materials from sediments and sludge or to separate useful components from debris. For example, in the petrochemical industry, solvents are used to remove impurities from gasoline by making them float or sink to the bottom for easy removal.
What are the two types of solvent extraction?
There are two main types of extraction: liquid-liquid extraction (also known as solvent extraction) and solid-liquid extraction. Both types of extraction are based on the same principle, which involves separating compounds based on their ability to dissolve in two different liquids or solid matter compounds that do not mix.
What are the common equipment used for solvent extraction?
The common equipment used for solvent extraction includes separatory funnels, Soxhlet extractors, and centrifuges.
What is solvent extraction?
Solvent extraction is a technique used to extract or separate a target compound from a mixture using a solvent.
What are the common applications of solvent extraction?
Solvent extraction is commonly used in industries such as petrochemical, pharmaceutical, and food processing to isolate, purify, or remove target compounds from a mixture.
What are the common types of solvent extraction?
The two common types of solvent extraction are liquid-liquid extraction and solid-liquid extraction. In liquid-liquid extraction, the target compound is extracted from a liquid phase using another immiscible liquid as the solvent. In solid-liquid extraction, the target compound is extracted from a solid phase using a liquid solvent.
What are the factors to consider when choosing a solvent for extraction?
The choice of solvent depends on the target compound’s properties, such as its solubility, polarity, and selectivity. Additionally, the solvent should be compatible with the sample matrix and have low toxicity and volatility.
What are the potential hazards associated with solvent extraction?
Solvent extraction can be hazardous due to the toxicity, flammability, and environmental impacts of the solvents used. It is important to follow proper safety procedures and use appropriate protective equipment when working with solvents.

 written by
written by 



Leave a Reply