Which Properties of Solvents are Useful for Solvent Extraction?
Selectivity of solvent, its Immiscibility with Water, boiling point, viscosity, Chemical Reactivity, density, and flash point are some properties that are useful for solvent extraction.
Also Read Factors That Affect Solvent Extraction
Properties of Solvents for Efficient Solvent Extraction
Here are the Properties of Solvents that are needed for Efficient Solvent Extraction:
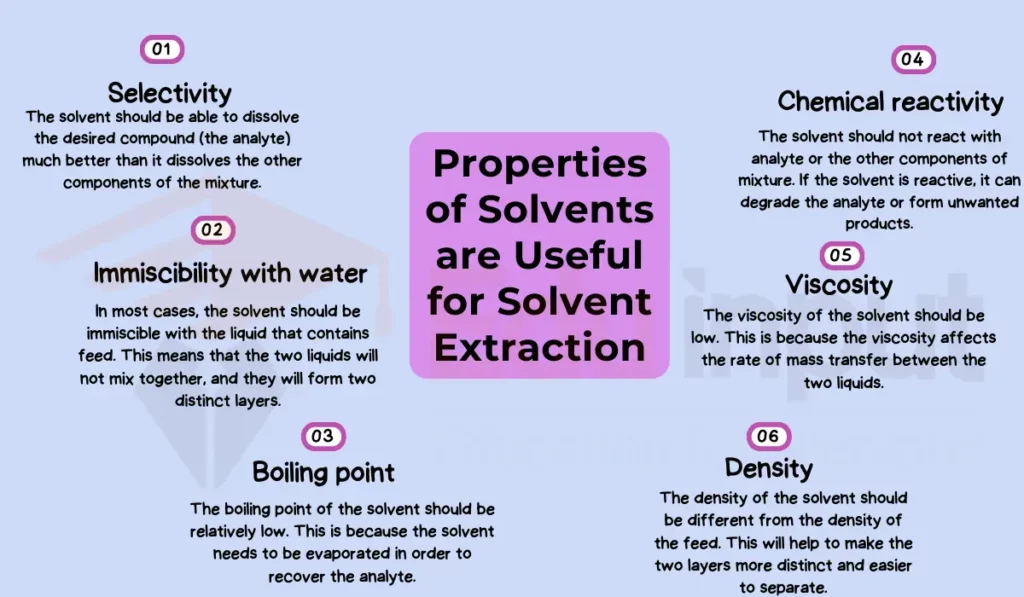
1. Selectivity
The selectivity of a solvent refers to its ability to preferentially dissolve the target compound over other components in a mixture. This relies heavily on the chemical affinity between the solvent and respective solutes.
A highly selective solvent will isolate the desired compound while leaving behind unextracted materials. Chloroform shows strong selectivity for caffeine extraction from coffee beans, attributed to intermolecular interactions between chloroform and caffeine.
2. Immiscibility with Water
Immiscibility is inability of a solvent to mix uniformly with water, instead separating into distinct solvent-rich and water-rich phases. This facilitates extraction of organic compounds from aqueous mixtures.
It simplifies the isolation of the solvent phase containing the dissolved product. Dichloromethane is commonly employed to extract non-polar lipids from biological fluids due to its immiscibility with water.
3. Boiling Point
A solvent’s boiling point directly impacts the ease of post-extraction processing. Solvents with low boiling points like hexane can be easily removed via evaporation. It leaves behind extracted material with no residual solvent. Hexane’s low boiling point enables efficient concentration of fragile natural products like essential oils and fragrances.
4. Chemical Reactivity
Reactivity considers how chemically inert a solvent is under extraction conditions, minimizing unwanted reactions with the solutes of interest. Reactions lead to losses in extract purity and quality. For example, ethanol shows excellent stability with sensitive cannabinoids during cannabis extractions to preserve medicinal properties.
5. Viscosity
Viscosity quantifies the thickness and flow characteristics of a liquid. In large-scale extraction processes, low viscosity solvents like toluene ensure adequate contact between solvent and feed material. This facilitates rapid mass transfer to improve extraction efficiency.
6. Density
A solvent’s density affects phase separation steps post-extraction. The organic phase needs to have an appropriately higher or lower density relative to the aqueous phase. In bitumen production from oil sands, toluene’s density causes it to sink below water layers, benefiting subsequent solvent recovery.
7. Flash Point
The flash point measures flammability levels. Depending on temperature settings, a higher flash point might be required for safe operation, especially in large, industrialized extraction equipment. Kerosene has found utility in supplemental oil extraction from depleted reservoirs due to relatively high flash points.
8. Toxicity
Toxicity levels require evaluation as more stringent environmental regulations are enacted. Bio-based greener solvents are being assessed to replace conventional options. Limonene obtained from orange peels demonstrates potential, with low toxicity coupled with strength in dissolving hydrophobic compounds.

 written by
written by 




Leave a Reply