Why Is Ethanol Good Solvent For Extraction?
Ethanol is a good solvent for extraction because it is relatively polar and can form strong intermolecular bonds with compounds in the solvent.
Ethanol is considered safe for use in food and pharmaceuticals when regulations are followed. Compared to many other organic solvents, ethanol is relatively less toxic and thus safer for extractions where the product will be consumed.
Also, Read Factors Affecting Solvent Extraction
Reasons Why Is Ethanol a Good Solvent For Extraction?
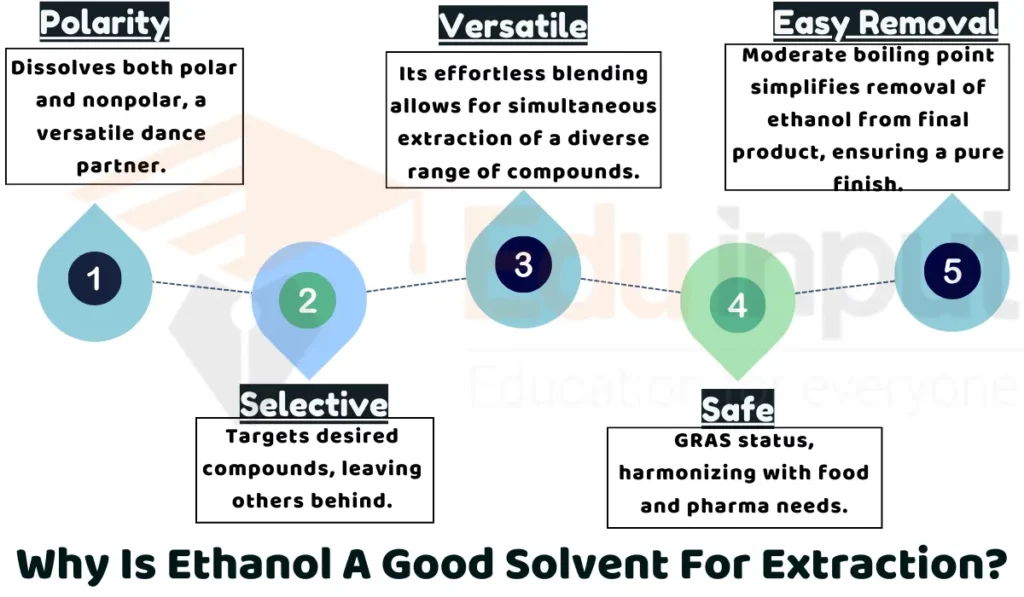
Here are the main Reasons Why Is Ethanol Good Solvent For Extraction:
1: Polarity
Ethanol molecules have both a positive and negative end. This allows ethanol to dissolve many examples of compounds, including ones that mix well with water and ones that don’t. This makes ethanol a very versatile solvent for extracting many different things from plants, like colors, oils, and plant chemicals.
2: Selectivity
Ethanol can be used to extract specific compounds from plants while leaving other compounds behind. By controlling temperature and time, researchers can fine-tune the ethanol extraction process to target only the desired chemicals. This selectivity allows them to obtain extracts enriched with certain ingredients.
3: Safety
Ethanol is considered safe for use in food and pharmaceuticals when following regulations. It is less toxic than some other solvents. This makes ethanol a safer choice for extractions, especially when the final product will be consumed or used as medicine.
4: Versatility
A major advantage of ethanol is that it mixes easily with water. This means ethanol can efficiently extract both water-soluble and alcohol-soluble ingredients in one step. This versatility allows ethanol to retrieve a very wide variety of plant compounds.
5: Solvent Removal
Ethanol has a relatively low boiling point compared to other solvents. This makes it easier to remove ethanol from the final botanical extract. For industries concerned about left-over solvents, ethanol’s easy removability is a major plus.
6: Economic Considerations
Ethanol is inexpensive and widely available. This affordability makes ethanol feasible for large commercial extraction facilities where cost-effectiveness is important.
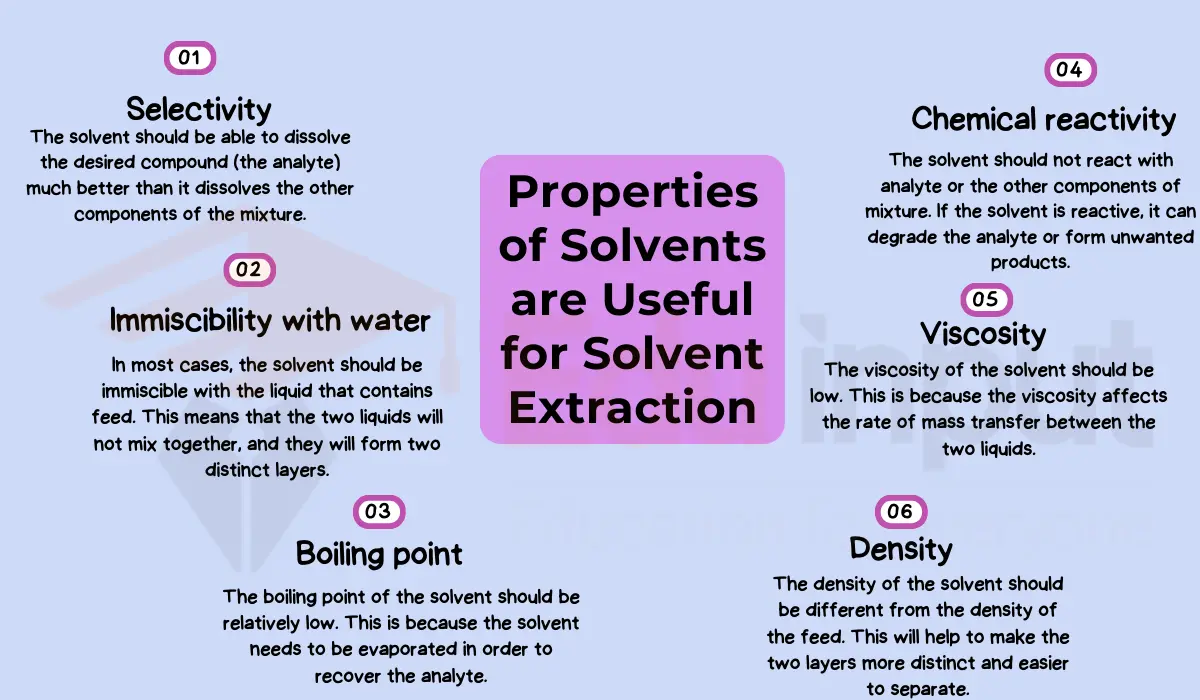
However, for some extractions alternative solvents may be better suited depending on the compounds of interest. While ethanol is versatile, it is not universally optimal. The specific needs of the process and properties of target compounds should guide solvent selection.





Leave a Reply