Why Glucose is Reducing Sugar?
Glucose is considered a reducing sugar because it has an aldehyde group (–CHO) in its molecular structure. The presence of this aldehyde group allows glucose to act as a reducing agent, which means it can donate electrons and reduce other compounds.
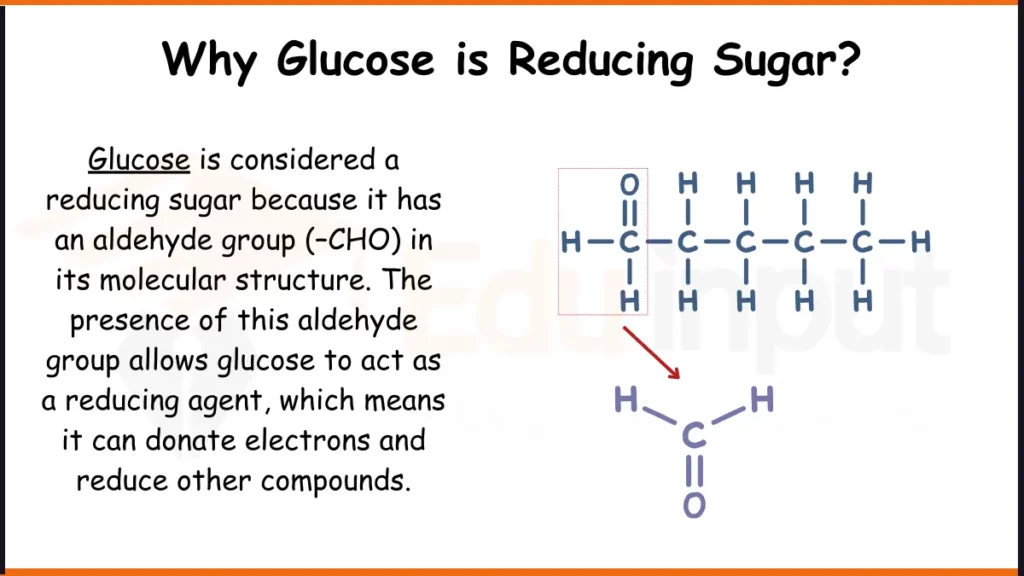
In a reducing sugar, the aldehyde group can undergo oxidation by losing hydrogen atoms (or electrons) and becoming a carboxyl group (–COOH). This oxidation reaction is facilitated by the presence of certain oxidizing agents or under specific conditions, such as heating with mild oxidizing agents like Benedict’s solution or Fehling’s solution, which are commonly used to test for the presence of reducing sugars.
Reasons Why Glucose is Reducing Sugar?
There are two main reasons why glucose is considered a reducing sugar:
1. Presence of free aldehyde group (-CHO)
The molecular structure of glucose contains a free aldehyde group (-CHO) at the first carbon atom. This aldehyde group is capable of donating electrons or hydrogen atoms, making glucose a reducing agent.
When the aldehyde group is oxidized, it loses hydrogen atoms and is converted into a carboxyl group (-COOH). This oxidation reaction is the basis for various tests used to detect the presence of reducing sugars, such as Benedict’s test or Fehling’s test.
2. Cyclic Structure With Hemiacetal Group
Glucose exists predominantly in a cyclic form, where the aldehyde group at carbon 1 forms a hemiacetal group (-CH(OH)-O-) by reacting with the hydroxyl group at carbon 5. This cyclic structure with a hemiacetal group also contributes to the reducing property of glucose.
In the cyclic form, the hemiacetal group can undergo a similar oxidation reaction as the aldehyde group, donating hydrogen atoms or electrons and becoming oxidized to an ester group (-C(=O)-O-).
The presence of either the free aldehyde group or the hemiacetal group in the cyclic structure allows glucose to act as a reducing agent. This is what makes Glucose a reducing sugar.
Are All Sugars Reducing Sugars?
No, not all sugars are reducing sugars. Sucrose (table sugar) and certain other disaccharides do not have a free aldehyde group and, therefore, are not classified as reducing sugars. However, when sucrose is hydrolyzed (broken down) into its component monosaccharides (glucose and fructose), it becomes a reducing sugar solution due to the presence of the aldehyde group in glucose.






Leave a Reply