Diffusion Explained – Definitions, Types, and Examples in Biology, Chemistry, and Physics
What is Diffusion?
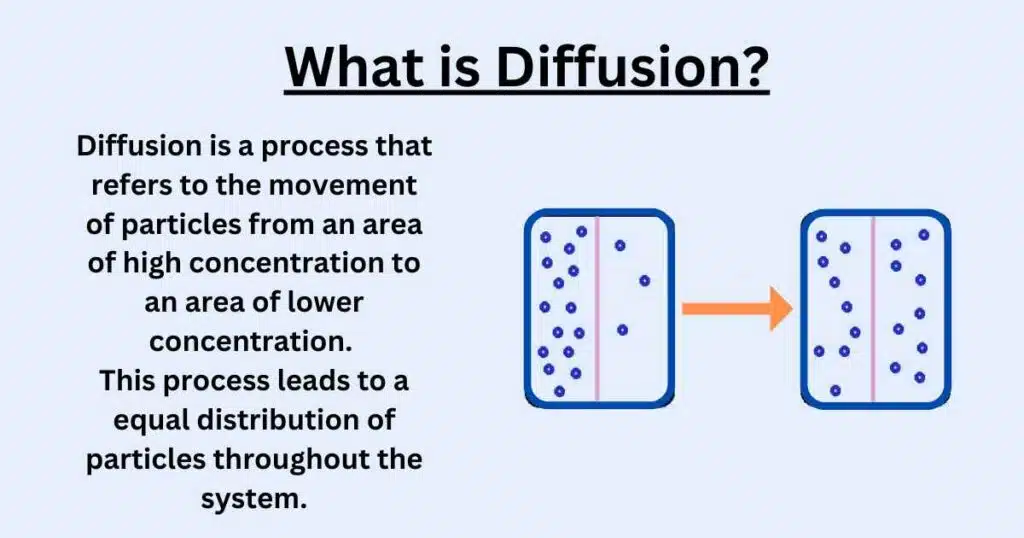
Diffusion is the movement of molecules from an area of high concentration to an area of low concentration, which is caused by their random motion. We can define diffusion as a movement that occurs due to the random kinetic energy of molecules, which causes them to spread out.
This definition of diffusion describes how particles naturally disperse to reach a state of equilibrium. The meaning of diffusion refers to this fundamental concept where molecules move across space until their concentration becomes uniform.
The definition of diffusion not only applies to scientific contexts but also provides insight into how materials naturally distribute themselves over time.
Diffusion Definition in Biology
In biology, diffusion is essential for transporting substances in and out of cells. Molecules like oxygen and carbon dioxide move across cell membranes from areas of higher concentration to lower concentration until equilibrium is reached.
The meaning of diffusion in biology highlights its importance in the transport of substances like nutrients, gases, and waste products within living organisms.
Diffusion Definition in Chemistry
In chemistry, diffusion is defined as the process through which molecules spread from an area of high concentration to an area of low concentration. Diffusion meaning in chemistry can be observed in various chemical reactions where molecules move to equalize concentration differences.
The diffusion in chemistry involves the spreading of molecules in a medium, which impacts reaction dynamics and the mixing of different chemicals.
In chemistry, diffusion influences the rate of chemical reactions. Different substances, whether solids, liquids, or gases, diffuse at different rates, affecting how quickly reactions occur.
Diffusion Definition in Physics
We can define diffusion In physics, as the process through which particles move or transfer energy, driven by random motion or gradients in concentration or temperature. For example, heat conduction involves thermal energy diffusing from warmer areas to cooler ones. This principle also applies to neutron diffusion in nuclear reactors.
In humans, movement of oxygen and carbon dioxide between the alveoli in the lungs and the surrounding capillaries occurs through diffusion. Oxygen diffuses from the alveoli into the blood, while carbon dioxide diffuses from the blood into the alveoli to be exhaled.
Read Difference Between Diffusion and Osmosis
Principles of Diffusion
Diffusion is how molecules naturally move and spread out in any substance. It happens because molecules are always moving randomly, bouncing around and bumping into each other. These molecules move from areas where they are crowded to places where there are fewer molecules.
No extra energy is needed for this movement. it just happens by itself. For instance, a tea bag in water – the color spreads out naturally without stirring. The bigger the difference in concentration between two areas, the faster molecules will move.
This movement keeps happening until the molecules are evenly spread out everywhere. Even when they are evenly spread, the molecules keep moving, but we don’t see any more changes because there are the same number of molecules everywhere. This process happens in gases, liquids, and even in our body’s cells.
Types of Diffusion
There are different types of diffusion that occur in various contexts. Each type plays an important role in biological, physical, and chemical processes. The classification of diffusion includes simple diffusion, facilitated diffusion, and osmosis, which represent three types of diffusion commonly observed in nature and scientific studies.
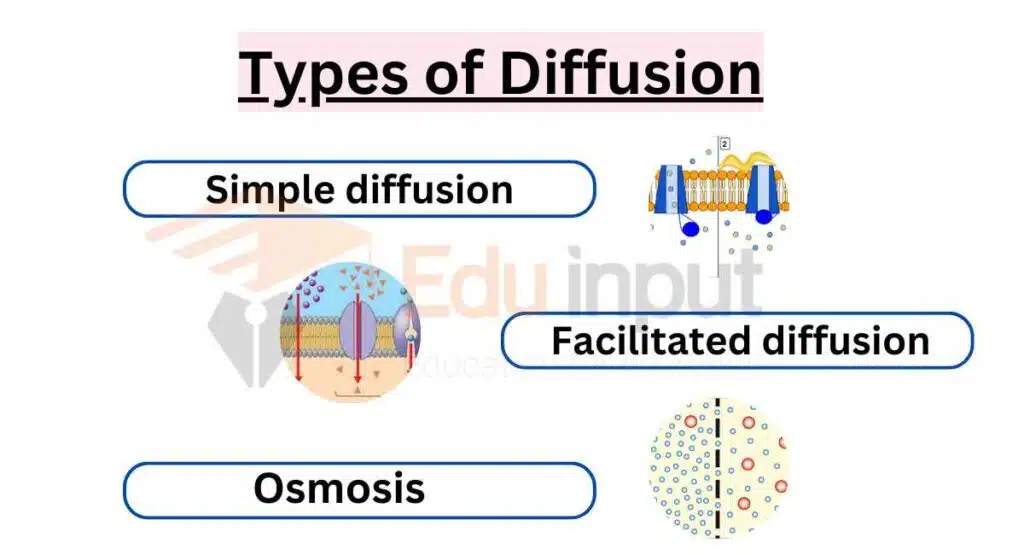
Simple diffusion
Simple diffusion is the movement of molecules from an area of high concentration to an area of lower concentration without the need for a membrane or carrier protein.
This type of diffusion is typically used for small nonpolar molecules, such as oxygen and carbon dioxide, which can diffuse easily through cell membranes via simple diffusion.
Among the types of diffusion, simple diffusion is often considered the most basic and universal, as it relies purely on the natural movement of molecules down their concentration gradient.
Facilitated diffusion
Facilitated diffusion involves the movement of molecules across a membrane from an area of high concentration to an area of lower concentration, with the help of a membrane protein.
This process is used for larger or polar molecules, such as glucose and amino acids, which cannot diffuse across the membrane through simple diffusion.
Facilitated diffusion shows how biological systems adapt to allow critical substances to enter or exit cells efficiently.
Osmosis
Osmosis is a special type of diffusion that refers to the movement of water molecules across a semi-permeable membrane from an area of low solute concentration to an area of high solute concentration.
This process is important in maintaining the proper balance of water and solutes in biological systems, such as the kidneys and plants.
Types of diffusion in biology, including simple diffusion and facilitated diffusion, are essential for the transport of molecules within cells. Similarly, the types of diffusion in physics highlight the broader principles of molecular movement that govern phenomena such as gas exchange and heat transfer. By examining what types of diffusion occur in various contexts, such as plants and other biological systems, scientists can better understand how substances move and interact.
Examples of diffusion in chemical and biological processes
Here are the examples of diffusion in Biology and Chemistry:
- Movement of gases through a membrane (e.g., oxygen and carbon dioxide exchange in the lungs)
- Mixing of solutions (e.g., sugar dissolving in water)
- Transport of nutrients in biological systems (e.g., glucose moving from the bloodstream into cells for energy production)
Diffusion Quiz (Diffusion Practice Questions)
1: What is diffusion?
(A) The movement of particles from low concentration to high concentration.
(B) The movement of particles from high concentration to low concentration.
(C) Active movement of particles, requiring energy from respiration.
Explanation: Diffusion moves particles from crowded areas to less crowded areas. It is a passive process, requiring no energy. This movement seeks equilibrium.
2: Which process is responsible for gas exchange in the lungs?
(A) Diffusion
(B) Osmosis
(C) Active transport
Explanation: Oxygen diffuses into blood, and CO₂ diffuses out. Gases move from high to low concentration. This is crucial for respiration.
3: What happens to the rate of diffusion as the temperature increases?
(A) It decreases.
(B) It increases.
(C) It stays the same.
Explanation: Heat speeds up particle movement. Faster movement results in faster diffusion. Kinetic energy increases with heat, leading to faster diffusion rates.
4: What happens to the rate of diffusion as the surface area increases?
(A) It increases.
(B) It decreases.
(C) It stays the same.
Explanation: More surface provides more space for particles to cross. Increased area results in increased diffusion. More area means more opportunity for movement.
5: Why do large multicellular organisms need transport systems while simple unicellular organisms do not?
(A) Large organisms have a much smaller surface area to volume ratio compared to smaller ones.
(B) Diffusion does not work in large multicellular organisms.
(C) Unicellular organisms have a much smaller surface area to volume ratio than multicellular organisms.
Explanation: Large organisms have less surface area per volume. Diffusion alone cannot supply all cells. They require transport systems for efficient distribution. Unicellular organisms rely solely on diffusion due to their size.
6: Transpiration is a phenomenon pertaining to
(A) Diffusion
(B) Osmosis
(C) Facilitated diffusion
(D) None of the above
Explanation: Transpiration is the diffusion of water vapor from plant leaves. Water moves from high to low concentration. This process is essential for plant water transport.
7: What is Graham’s law of diffusion?
(A) Rate is directly proportional to density.
(B) Rate is directly proportional to the square of density.
(C) Rate is inversely proportional to the square roots of densities.
(D) Rate is directly proportional to temperature.
Explanation: Denser gases diffuse slower than lighter ones. This law relates diffusion rate to gas density. It is an inverse relationship.
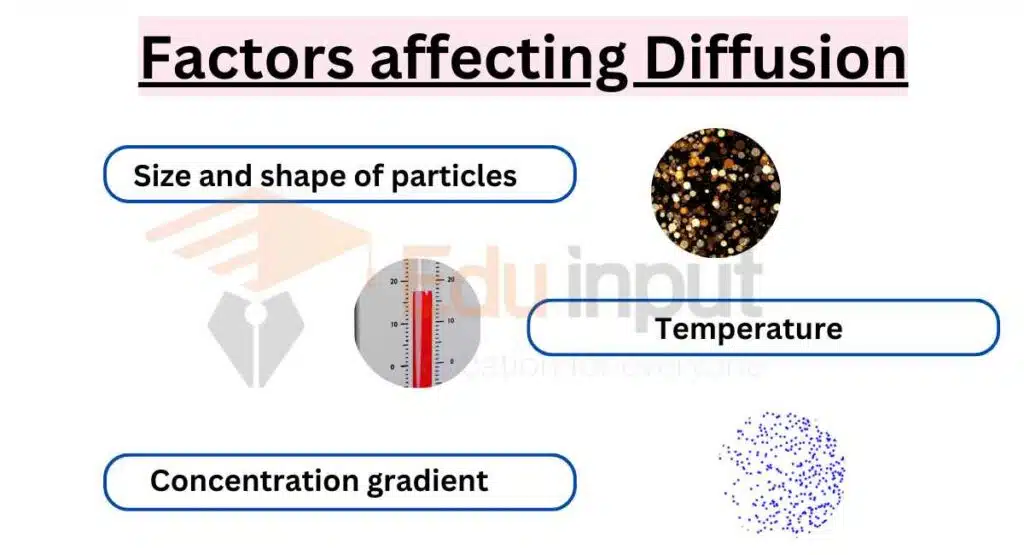
8: What are the various factors that affect the rate of diffusion?
(A) Only pressure and volume.
(B) Only particle size.
(C) Only temperature.
(D) Temperature, particle size, area, gradient.
Explanation: Temperature, particle size, surface area, and concentration gradients impact diffusion. Each factor directly influences particle movement. Steep gradients and high temperatures speed up diffusion.
9: Which of the following is NOT a type of passive transport?
(A) Diffusion
(B) Osmosis
(C) Endocytosis
(D) Facilitated diffusion
Explanation: Endocytosis requires cell energy to engulf substances. It is active, not passive. Passive transport does not require energy input from the cell.
10: Chamber A contains 40% helium, and Chamber B contains 20% helium. Chambers are connected by a tube, and molecules are free to cross. Which of the following will occur?
(A) Some helium will move from Chamber A to Chamber B.
(B) Some helium will move from Chamber B to Chamber A.
(C) Helium will remain concentrated in Chamber A.
(D) All of the helium will move into Chamber B.
Explanation: Helium moves from high to low concentration. Gases diffuse to equalize distribution. This movement follows the concentration gradient.
11: What will happen to an animal cell placed in a saltwater solution?
(A) The cell will shrink.
(B) The cell will expand.
(C) The cell will burst.
(D) The cell will shrink and then expand and then shrink again.
Explanation: Salt water pulls water out of the cell. The cell loses water and shrivels. High solute concentrations cause water to exit the cell.
12: An animal cell placed in a hypotonic solution will:
(A) Die
(B) Take on water
(C) Lose water
(D) Divide
Explanation: More water exists outside the cell. Water flows into the cell, causing it to swell. Hypotonic solutions have lower solute concentrations outside the cell.
Also Learn About:
FAQs
What is the definition of diffusion?
Diffusion is a process of movement of molecules (solids, liquids, gases) from higher concentration to lower concentration.
Why is it called diffusion?
The word “Diffusion” was derived from a Latin word “diffusionem, diffusio” meaning “to spread out/or pouring forth”. That’s why it is called diffusion as solute molecules spread out in solvent.
Who discovered diffusion?
Thomas Graham discovered the phenomenon of diffusion in gases for the first time.



Leave a Reply