Co-ordinate covalent bond, definition, types, examples, Properties and factors
Co-ordinate covalent bond definition
A bond formed by sharing of an electron pair that is donated by one of the two bonded atoms is called a coordinate covalent bond.
Explanation
The atom, ion, or molecule that donates an electron pair is called a donor and that which accepts a pair of electrons is called an acceptor. It is represented by an arrow (→) pointing from donor to acceptor. The bond formed between an acceptor species is called a coordinate covalent bond.
Coordinate covalent bonds represent a captivating dimension in the realm of chemical bonding. Their asymmetrical nature and dynamic electron sharing make them pivotal in the formation of complex molecular structures, especially in coordination compounds. Understanding the definition, types, formation, characteristics, and factors influencing coordinate covalent bonds is key to unraveling the intricacies of chemical interactions in diverse compounds and applications.
Types of Coordinate Covalent Bonds
Monodentate Bonds
In these bonds, the donating atom provides only one electron pair.
Example: Ammonia (NH₃) acting as a ligand donating a single pair of electrons to a metal cation
Bidentate Bonds
The donating atom provides two electron pairs.
Example: Ethylenediamine which has two amine groups, each donating a lone pair of electrons to form a bidentate bond with a metal cation.
Tridentate Bonds
Three electron pairs are provided by the donating atom.
Example: Triethylenetetramine is a molecule with three amine groups, each capable of forming a coordinate covalent bond.
Multidentate Bonds
Donating atoms can contribute to multiple electron pairs.
Example: Ethylenediaminetetraacetate a common multidentate ligand with four donor sites (two nitrogen and four oxygen atoms).
Examples
i) Bond Formation between NH3 and BF3
Nitrogen in NH3, has one lone pair of electrons, whereas B in BF, is electron deficient. So boron can accept a lone pair of electrons. This results in the formation of a coordinate covalent bond.
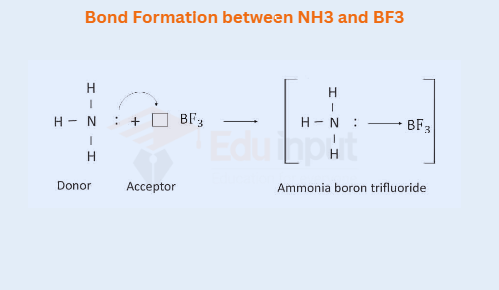
Just like BF3, compounds like AlCl3, AlBr3, etc. make the coordinate covalent bond with ammonia.
ii) Formation of ammonium ion
After the formation of a coordinate covalent bond, there remains no difference between a covalent and a coordinate covalent bond. It is observed experimentally that all the bonds of NH4+ are equivalent. Similarly, P+H4 has one coordinate covalent bond.

iii) Formation of Hydronium ion
Important derivatives of ammonia like primary, secondary, and tertiary amines are also protonated due to the formation of coordinate covalent bonds.

Alcohols and ketones have also electron pairs on the oxygen atom so they accept the protons to form coordinated covalent bonds.
Co-ordinate covalent bonds are also formed in HNO3, H2SO4, HCIO2, HCIO3, and HClO4.
In oxy acids of chlorine, the coordinate covalent bond is between Cl-atom and O-atom.
Properties of Coordinate Covalent Compounds:
Coordinate covalent compounds exhibit characteristics that are similar to covalent compounds:
1. They do not ionize in water.
2. They are poor conductors of electricity.
3. They are very sparingly soluble in water but dissolve in organic solvents.
4. Since a coordinate covalent bond is semi-polar, the coordinate compounds possess melting and boiling points that are higher than those of purely covalent compounds but lower than ionic compounds.
5. The coordinate covalent bond is also rigid and directional and therefore, such compounds can also exhibit space isomerism.
6. The coordinate covalent bond is easily broken when the donor and acceptor are such molecules that are capable of independent existence.
Factors Influencing Coordinate Covalent Bonding
Lewis Acid-Base Interaction
The presence of a Lewis acid and a Lewis base is essential for the formation of coordinate covalent bonds.
Electronegativity Difference
The electronegativity difference between the donating and accepting atoms influences the strength and polarity of the bond.
Orbital Overlap
Efficient overlap of atomic orbitals enhances the stability of coordinate covalent bonds.
Charge Distribution
The distribution of charge on the atoms involved affects the nature of the bond and the resulting molecular structure.

 written by
written by 


Leave a Reply