Hybridization, definition, types and significance
Definition
The process in which atomic orbitals of different energies and different shapes intermix to form an equal number of orbitals, which are identical in all respects like shape, angle, length, and energy except orientation in space, is called hybridization.
Explanation
Hybridization is a concept in chemistry that describes the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. This process occurs when an atom is preparing to bond with other atoms to create molecules. Hybridization provides a more accurate description of molecular geometries and explains certain observed properties.
The nature and shapes of the orbitals in the outermost shell of an atom mix up with each other. In this, way they get the stability by rearranging the geometries of the orbitals. For this purpose, they are mixed up in definite ratios to give new hybrid orbitals. The bonds so produced are comparatively stable.
Need for hybridization
We have studied overlapping between unmodified (unhybridized) atomic orbitals in valence bond theory. There are some problems in explaining the formation of bonds and the geometry of molecules. For example, the bond angles of CH4, NH3, and H2O can be explained based on the concept of hybridization.
Change of Valency and hybridization
Hybridization explains the valency of elements. In hybridization, the first step is usually forming an excited state. It involves the unpairing of electrons followed by the promotion of an electron to a higher energy level. As a result, there is an increase in the number of unpaired electrons. So the valency of the element changes as explained in the following table:
The energy of excitation
The energy required for this excitation comes from the energy that is released during the process of hybridization and bond formation with other atoms. Remember that excitation of electrons and hybridization are simultaneous processes.
Types of hybridization
Hybridization involves the mixing of atomic orbitals to form new hybrid orbitals, leading to the creation of stronger and more directional bonds in molecules. The types of hybridization are classified based on the combination of atomic orbitals involved. Here are some common types of hybridization:
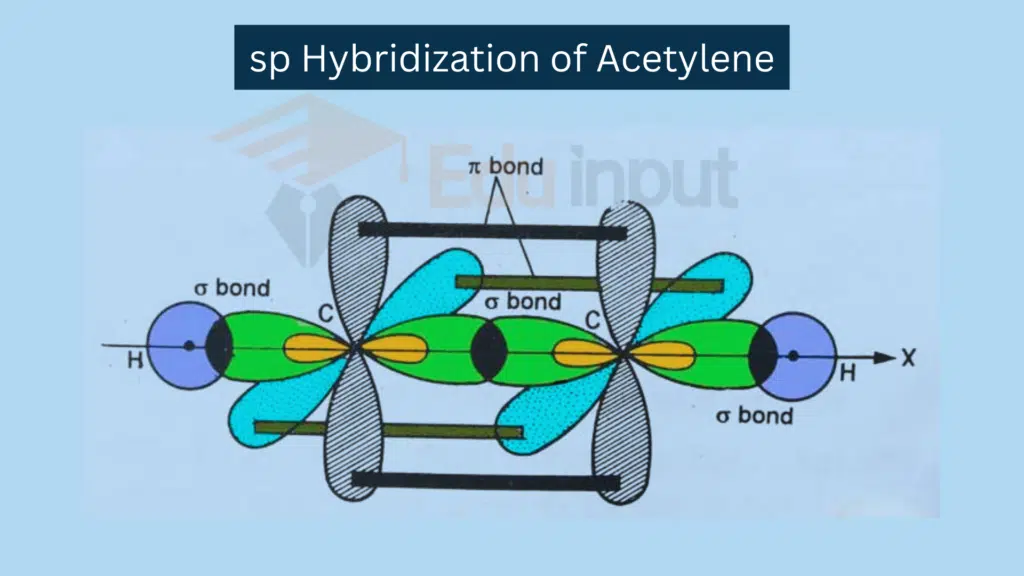
sp Hybridization
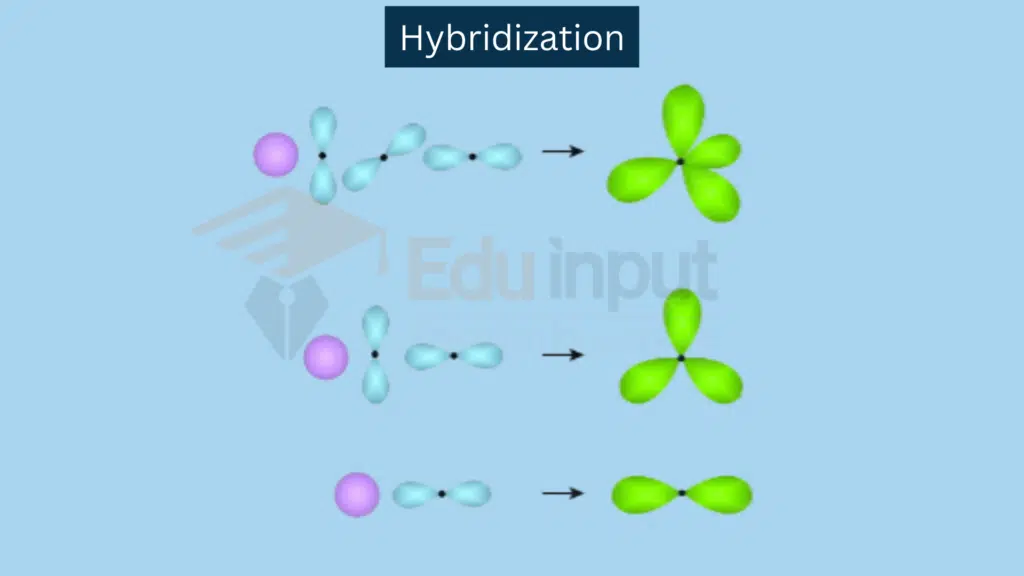
sp hybridization involves a combination of one s and one p orbital.
Combination: One s orbital and one p orbital combine.
Resulting Hybrid Orbitals: Two sp hybrid orbitals.
Example: Linear molecules like C2H2.
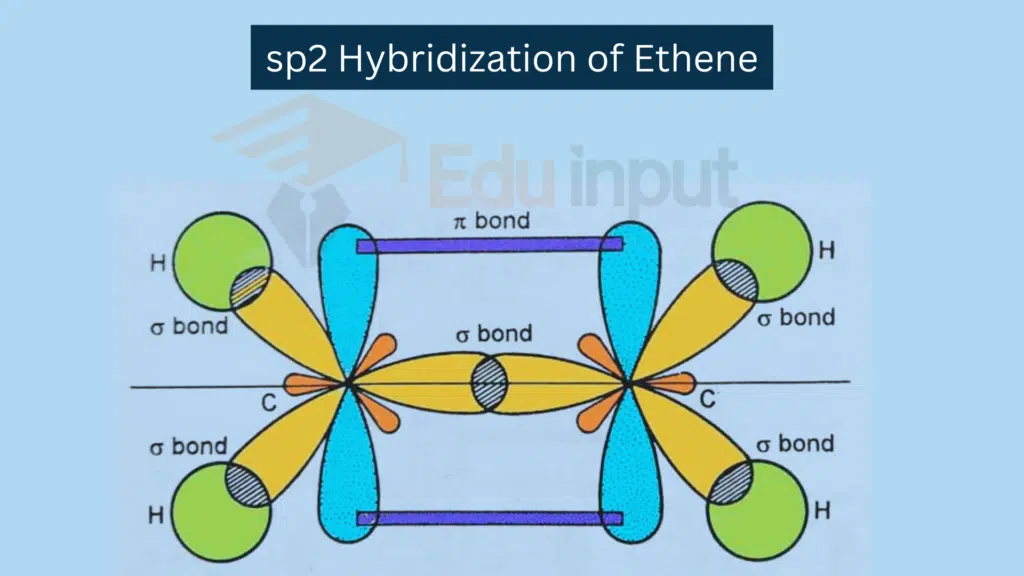
sp² Hybridization
sp² Hybridization involves a combination of one s orbital and 2 p orbitals.
Combination: One s orbital and two p orbitals combine.
Resulting Hybrid Orbitals: Three sp² hybrid orbitals.
Example: Trigonal planar molecules like C2H4.
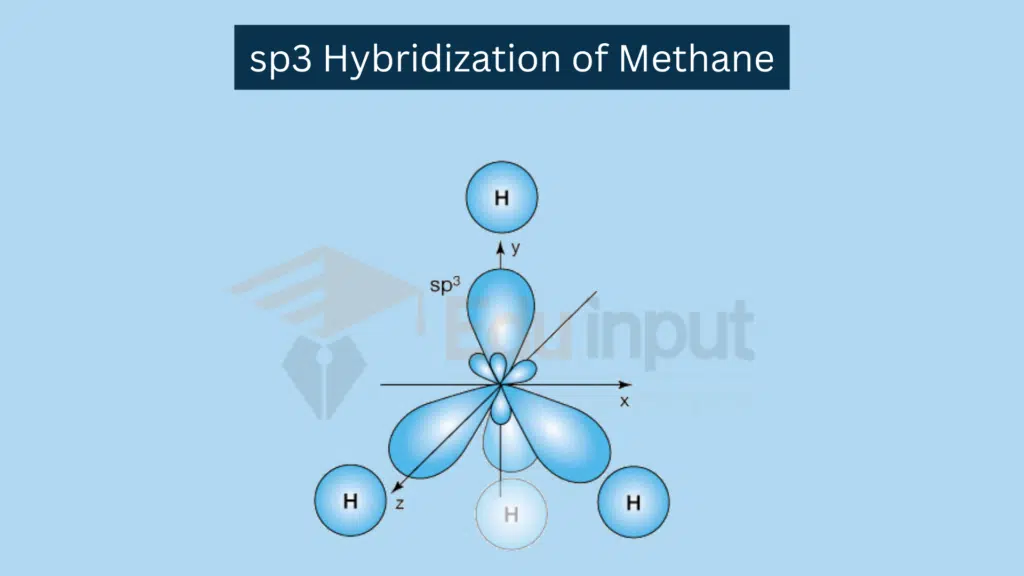
sp³ Hybridization
sp³ Hybridization involves combination of one s orbital and three p orbitals.
Combination: One s orbital and three p orbitals combine.
Resulting Hybrid Orbitals: Four sp³ hybrid orbitals.
Example: Tetrahedral molecules like CH₄.
dsp³ Hybridization
Combination: One s orbital, three p orbitals, and one d orbital combine.
Resulting Hybrid Orbitals: Five dsp³ hybrid orbitals.
Example: Trigonal bipyramidal molecules like PCl₅.
d²sp³ Hybridization
Combination: One s orbital, three p orbitals, and two d orbitals combine.
Resulting Hybrid Orbitals: Six d²sp³ hybrid orbitals.
Example: Octahedral molecules like SF₆.
sp³d Hybridization
Combination: One s orbital, three p orbitals, and one d orbital combine.
Resulting Hybrid Orbitals: Five sp³d hybrid orbitals.
Example: Trigonal bipyramidal molecules with one lone pair, like SF₄.
sp³d² Hybridization
Combination: One s orbital, three p orbitals, and two d orbitals combine.
Resulting Hybrid Orbitals: Six sp³d² hybrid orbitals.
Example: Octahedral molecules with two lone pairs, like SF₄²⁻.
Significance of hybridization
Hybridization gives an entirely new shape and orientation to the valence orbitals of an atom. It is quite significant in the determination of the geometry of the molecules.
Hybridization is a concept in chemistry that plays a significant role in explaining molecular geometry, bond angles, and the nature of chemical bonds. Here are some key aspects of the significance of hybridization:
Molecular Geometry and Bond Angles
Explanation of Molecular Shapes: Hybridization helps explain the observed molecular shapes. By combining atomic orbitals to form hybrid orbitals, the geometry of molecules can be rationalized.
Consistency with Experimental Observations
Hybridization predictions often align with experimental observations of bond angles and molecular shapes, providing a theoretical framework for understanding molecular structures.
Stability of Molecules
Enhanced Bonding Strength: Hybridization allows for the formation of stronger and more stable bonds. Hybrid orbitals with directional characteristics result in better overlap with neighboring orbitals, leading to more effective bonding.
Explanation of Double and Triple Bonds
Unification of Sigma (σ) and Pi (π) Bonds: Hybridization provides a unified approach to understanding both sigma and pi bonds. For example, in molecules with double or triple bonds, hybridization explains the formation of sigma and pi bonds through the overlap of hybrid orbitals.
Facilitation of Reaction Mechanisms
Prediction of Reactivity and Mechanisms: Understanding the hybridization of atoms in reactants helps predict the reactivity of molecules and the mechanisms of chemical reactions. This is particularly useful in organic chemistry.
Adaptation to Electron Pair Repulsion
Minimization of Electron Pair Repulsion: Hybridization allows for the arrangement of electron pairs around a central atom in a way that minimizes repulsion. This is per the Valence Shell Electron Pair Repulsion (VSEPR) theory.
Explanations in Coordination Chemistry
In coordination complexes involving transition metals, hybridization is crucial in explaining the nature of metal-ligand bonds and the geometry of the complexes.
Insights into Molecular Spectra
Hybridization concepts contribute to the interpretation of molecular spectra, especially in the context of electronic transitions and vibrational modes.
Prediction of Properties
Hybridization allows chemists to predict certain properties of molecules, such as dipole moment, based on the arrangement of hybrid orbitals and the nature of bonding.
Foundation for Molecular Orbital Theory
Hybridization serves as a foundational concept for understanding Molecular Orbital Theory. It provides a step toward a more advanced understanding of electron distribution in molecules.






Leave a Reply