Shapes of Atomic orbitals-Orientation of s, p, d, f orbitals in space
Whenever we talk about the shapes of orbitals we talk about the angular probability distribution curves. These are the space orientation or boundary surface of orbitals.
These curves tell us how the probability of finding the electron varies with its direction from the nucleus without any reference to its distance from the nucleus.
In other words, these curves show us how the various orbitals of a given sub- shell are disposed along the x, y, and z-axis. Let’s discuss the shapes, space orientation, and electron density orbitals.
Shapes of Atomic orbitals
Here are the different Shapes of Atomic orbitals:
1. S orbital
As the diagram of the s orbital shows that the probability of finding the electron in all the directions from the nucleus is the same. So s orbital is spherical and symmetrical in shape. As the value of the principle, the quantum number increases the size of s- orbital also increases.

2. P sub-shell
The magnetic quantum number for p sub- shell has three values i.e. (-1, 0, +1) so it has three space orientations, px (m= 0), py(m= +1), Pz(m= -1). All three orbitals have a dumb-bell shape. Each p orbital has two egg-shaped lobes. These orbitals have directional character.

3. d sub-shell
The value of magnetic quantum numbers for the d sub-shell is (0, +1, +2), so it has five space orientations. They are equivalent in energy so long as it is not under the influence of a magnetic field.
They are said to be five-fold generate. They are designated as dxy, dyz, dzx – y2, and dz2 and have complex geometrical shapes. The conventional boundary surfaces or shapes are shown in the image below.


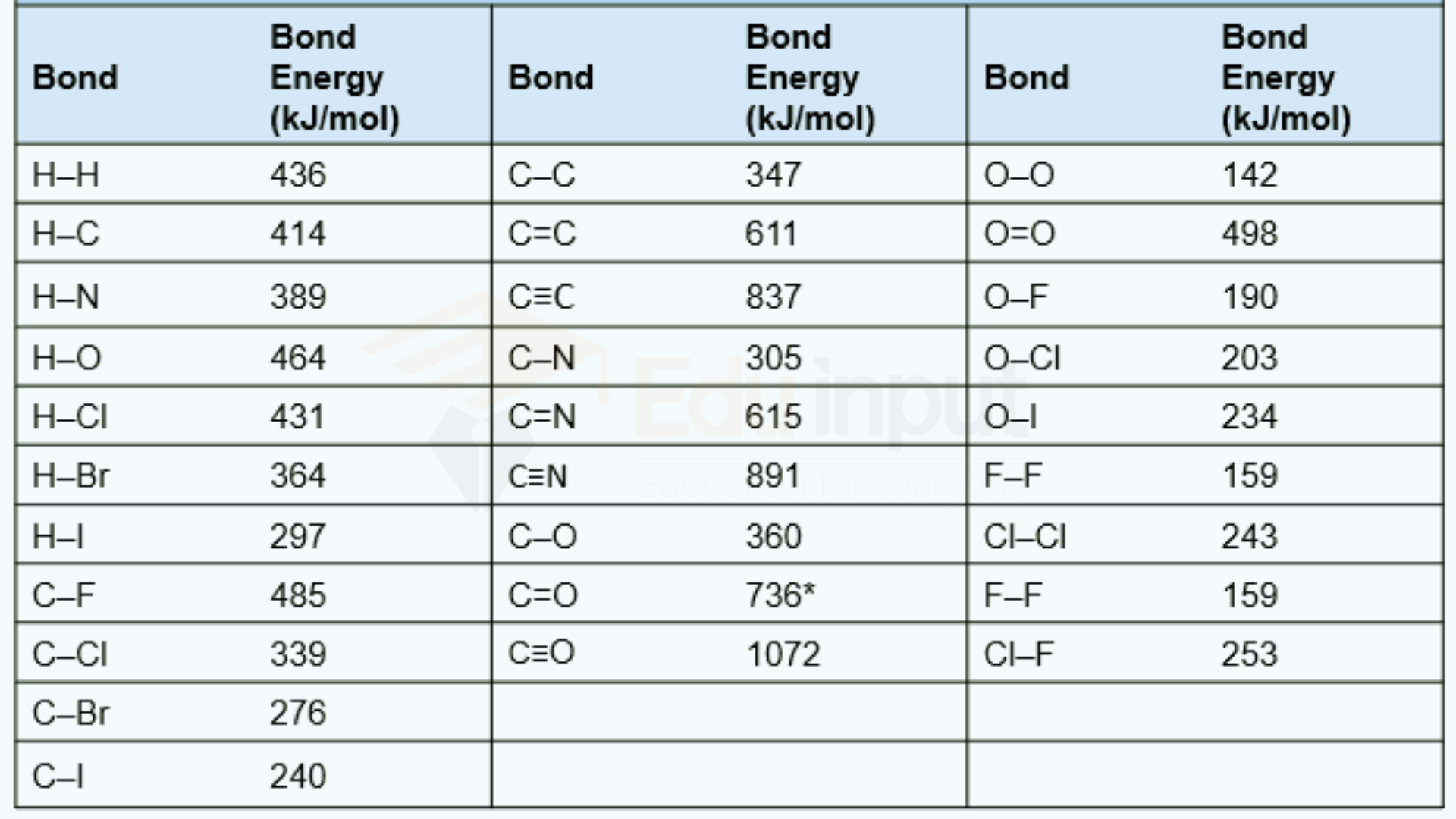



Leave a Reply