Aufbau Principle-Definition, characteristics, diagram, exception
Electrons will fill the lowest energy electron shells in a neutral atom according to the Aufbau principle. From the lowest energy to the highest energy, the electrons fill the orbitals. The electronic structure of an atom can be determined with the help of the Aufbau principle.
The Bohr model shows that electrons fill the lowest available energy level before starting to fill the next shell. The principle was called the Aufbau principle. The principle of the Aufbau means building up in German, and it explains how the electron shells are built up.
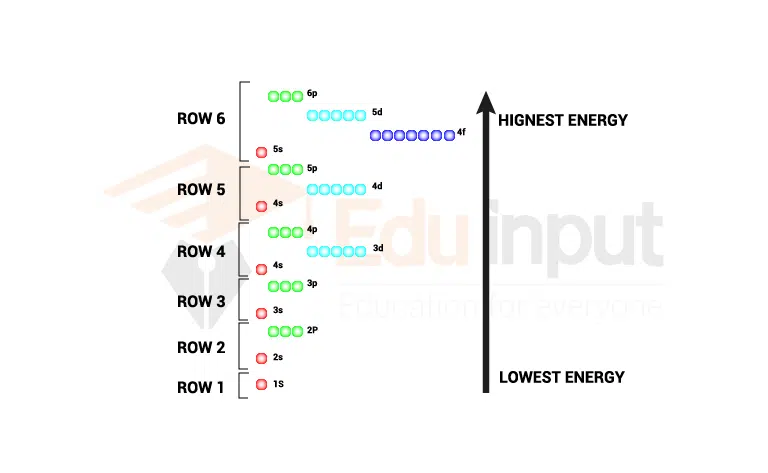
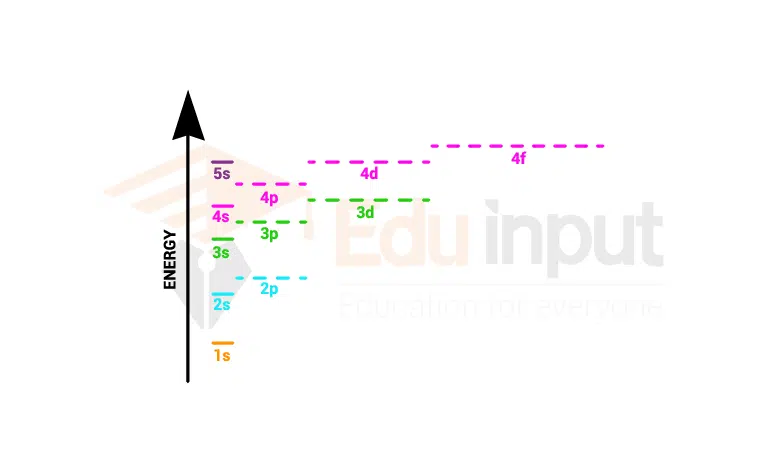
Below is a diagram illustrating the order in which atomic orbitals are filled. The azimuthal quantum number is referred to as ‘n’ and ‘l’ referred to as quantum numbers ( Principal & quantum).

It is also to note that this rule follows other rules of electron distribution such as Pauli exclusion and Hund’s rule.
Order of filling electrons in shells as per Aufbau Rule
Follow image makes the concept clear how electrons are filled in shells according to the Aufbau rule.
Characteristics of the Aufbau principle
The principle states that electrons first go to the ones with the lowest energy. The electrons enter the orbitals with higher energies when the lower ones are filled.
The (n+l) rule can be used to determine the order in which the energy of orbitals increases. The lower the values, the lower the energies. The lower the n value, the lower the energy associated with it.
The order in which the orbitals are filled with electrons is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.
Limitations of Auf bau principal
The principle is not universally accepted. Some atoms don’t obey it, such as transition metals and lanthanides.
The Aufbau principle suggests that the electron configuration of chromium is [Ar]3d54s1 and not [Ar]3d44s2. The increased stability provided by half-filled subshells and the relatively low energy gap between the 3d and the 4s subshells is some of the factors attributed to this exception.



Leave a Reply